Hydrocarbons (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Saturated & unsaturated compounds
What are hydrocarbons?
Hydrocarbons are compounds that contain only carbon and hydrogen atoms
They can be classified into two main types based on the bonding between their carbon atoms
1. Saturated hydrocarbons
A hydrocarbon is saturated if the carbon atoms are joined together by single C-C bonds only
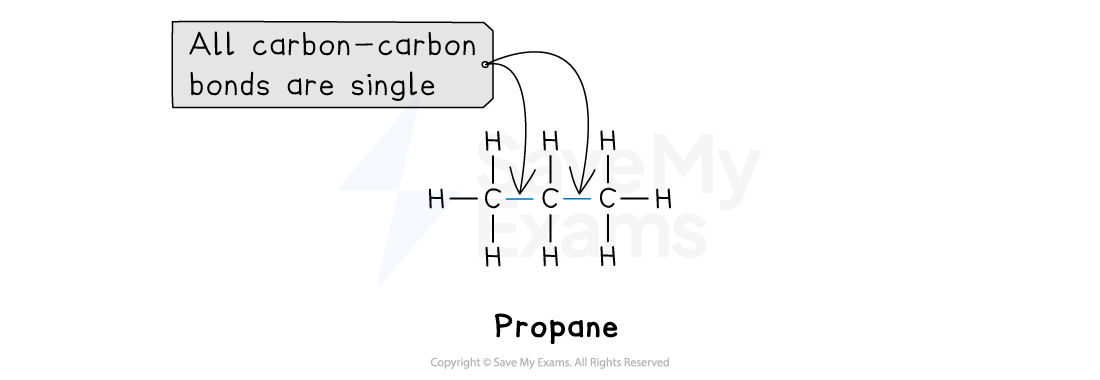
The two homologous series of saturated hydrocarbons you need to know are:
2. Unsaturated hydrocarbons
A hydrocarbon is unsaturated if it contains at least one double C=C bond
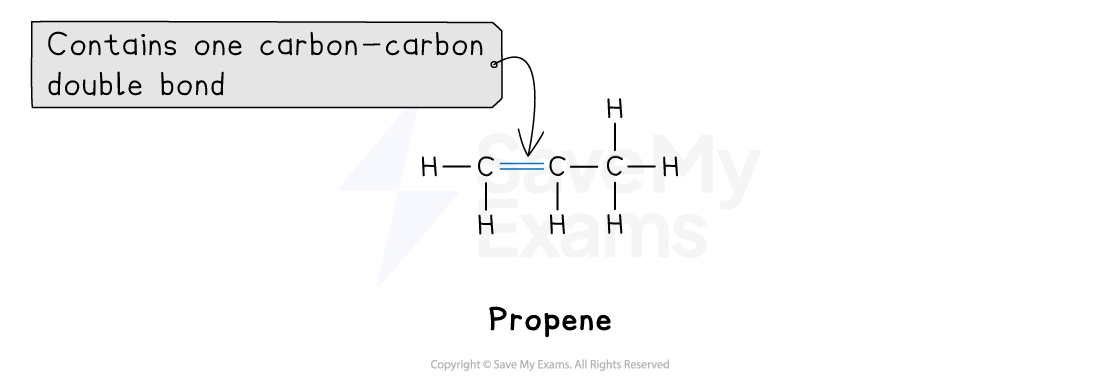
The homologous series of unsaturated hydrocarbons you need to know is the alkenes
Unsaturation testing - the bromine solution test
All alkanes are saturated and alkenes are unsaturated
The presence of the C=C double bond allows alkenes to react in ways that alkanes cannot
This means that a simple chemical test can distinguish between a saturated and an unsaturated hydrocarbon
Bromine solution is used in the test for alkenes as it is safer and easier to handle than bromine
A simple chemical test can tell the difference between a saturated and an unsaturated hydrocarbon.
Method
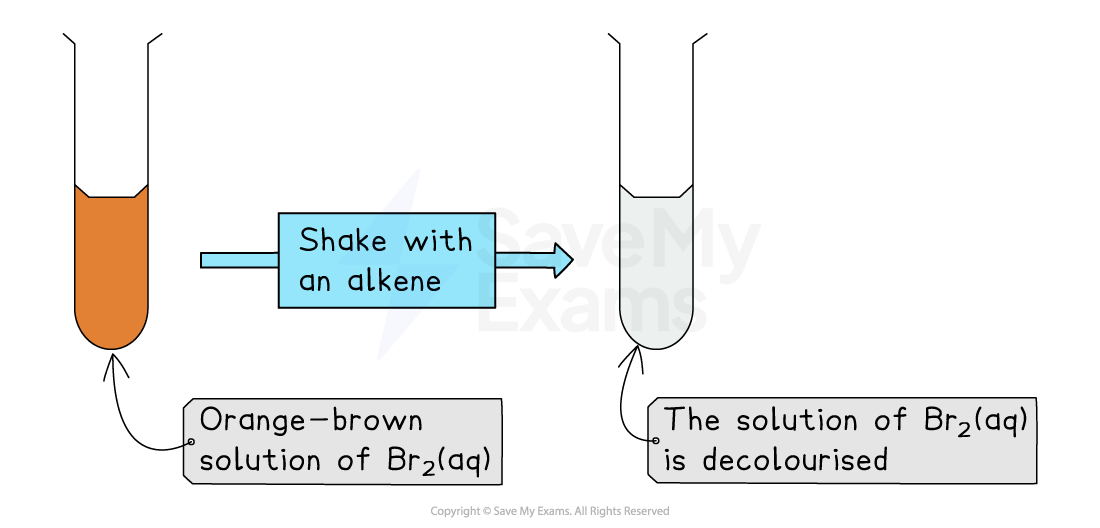
Place a few drops of hydrocarbon in a test tube
Add a few drops of orange-brown bromine solution to the test tube
Add a bung / stopper
Gently shake the test tube
The results
Unsaturated compounds, e.g., alkenes, decolourise the bromine solution
It turns from orange-brown to colourless
Saturated compounds, e.g. alkanes, show no visible change
The mixture remains orange/brown
Examiner Tips and Tricks
Describing the result is a very common place to lose marks.
Do not say:
"The alkene decolourises bromine"
"It goes clear"
To get the mark, you must describe what you see, for example:
"The bromine solution is decolourised"
"The orange-brown colour disappears"
"It turns colourless"
Explaining the results
The alkene performs an addition reaction
This is where the C=C double bond breaks and the bromine atoms add onto the carbon atoms
This uses up the bromine
So, the colour disappears
Saturated compounds cannot do addition reactions

Unlock more, it's free!
Was this revision note helpful?
