Structural Formulae (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Full & shortened structural formulae
Organic chemistry uses different types of formulae to represent molecules:
Molecular formula
This shows the total number of each type of atom
e.g., butane is written as C4H10
It tells you what's in a molecule, but not how it's connected
Full structural formula
This is a diagram that shows every atom and every single bond
This is the most detailed picture
Shortened structural formula
This is a line of text that summarises the structure
It groups the atoms on each carbon together
Condensed structural formula
This is a common way of writing a formula that gives some structural information
It shows the functional group separately
e.g., ethanol is written as C2H5OH
Example of different formulae
Let's look at an example for the alkane, butane:
Its molecular formula is C4H10
This shows that butane contains 4 carbon atoms and 10 hydrogen atoms
To write its shortened structural formula:
We write each carbon and its attached hydrogens in order
The first carbon is bonded to 3 hydrogen atoms and 1 neighbouring carbon atom
The second carbon atom is bonded to 2 hydrogen atoms and 2 neighbouring carbon atoms
The third carbon atom is bonded to 2 hydrogen atoms and 2 neighbouring carbon atoms
The final carbon is bonded to 3 hydrogen atoms and 1 neighbouring carbon atom
So, the shortened structural formula is CH3CH2CH2CH3
To draw its full structural formula:
Draw out every atom and every bond
So, the full structural formula is:

Examiner Tips and Tricks
The "Four Bond" rule
When drawing a full structural formula, always check your final drawing
Every carbon atom must have exactly four bonds
Examples from each homologous series
Alkanes
Alkanes are saturated hydrocarbons containing only C-C single bonds
Their shortened structural formula is the most common way to write them
They have no specific functional group to highlight
Name | Molecular formula | Full structural formula | Shortened structural formula |
|---|---|---|---|
Methane | CH4 |  | CH4 |
Ethane | C2H6 |  | CH3CH3 |
Propane | C3H8 |  | CH3CH2CH3 |
Alkenes
Alkenes are unsaturated hydrocarbons containing at least one C=C double bond
This double bond is their functional group
Name | Molecular formula | Full structural formula | Shortened structural formula |
|---|---|---|---|
Ethene | C2H4 |  | CH2CH2 |
Propene | C3H6 |  | CH2CHCH3 |
But-1-ene | C4H8 | 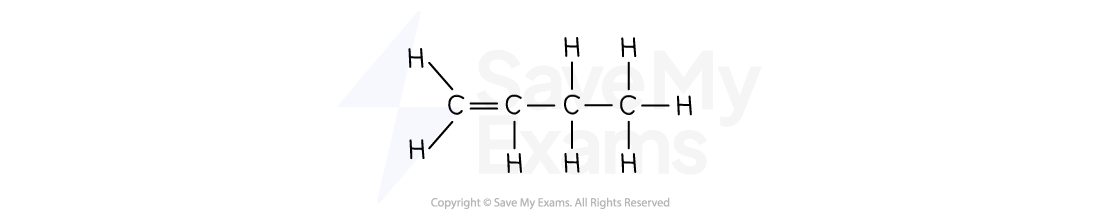 | CH2CHCH2CH3 |
Cycloalkanes
Cycloalkanes are saturated hydrocarbons where the carbon atoms form a ring
Their full structural formula is the most important representation
Shortened structural formulae are not usually required for cycloalkanes
Name | Molecular formula | Full structural formula |
|---|---|---|
Cyclopropane | C3H6 |  |
Cyclobutane | C4H8 |  |
Cyclopentane | C5H10 | 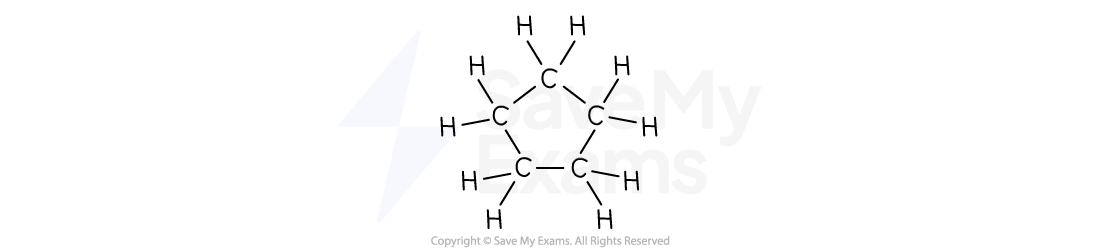 |
Worked Example
Draw the full structural formula for cyclohexane, C6H12
Answer:
From the name:
Cyclo- means it's a ring
hex- means 6 carbon atoms
-ane means all C-C single bonds
To draw the full structural formula:
Draw a hexagon of 6 carbon atoms
Add bonds and hydrogen atoms to each carbon until every carbon has a total of 4 bonds

Alcohols
Alcohols are a homologous series containing the -OH (hydroxyl) functional group
Since this group is so important, their formulae are often written as a condensed formula to show it
Name | Molecular formula | Condensed structural formula | Full structural formula | Shortened structural formula |
|---|---|---|---|---|
Methanol | CH4O | CH3OH |  | CH3OH |
Ethanol | C2H6O | C2H5OH |  | CH3CH2OH |
Propan-1-ol | C3H8O | C3H7OH |  | CH3CH2CH2OH |
Worked Example
Write the shortened structural formula for butan-1-ol
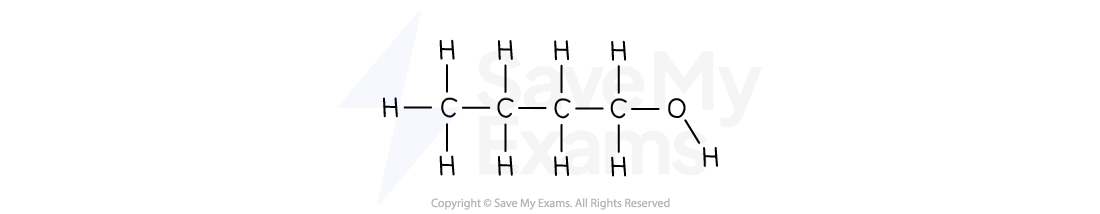
Answer:
From the name:
But- means a 4-carbon chain
-ol means an -OH group
-1- means the -OH is on the first carbon
To write the shortened structural formula:
Start with the first carbon and its group:
CH2OH
Add the two middle carbons:
CH2CH2
Add the carbon:
CH3
Combine them in order:
CH3CH2CH2CH2OH
Carboxylic acids
Carboxylic acids contain the -COOH (carboxyl) functional group
Like alcohols, their formulae are often written in a condensed way to highlight this important functional group
Name | Molecular formula | Condensed structural formula | Full structural formula | Shortened structural formula |
|---|---|---|---|---|
Methanoic acid | CH2O2 | HCOOH |  | HCOOH |
Ethanoic acid | C2H4O2 | CH3COOH |  | CH3COOH |
Propanoic acid | C3H6O2 | C2H5COOH |  | CH3CH2COOH |
Worked Example
Draw the full structural formula for butanoic acid, C3H7COOH
Answer:
From the name:
Butan- means 4 carbons in total
-oic acid means it has a -COOH group
To draw the full structural formula:
Draw the carboxyl group (-COOH) at the end of the chain
Draw the other 3 carbons in a chain leading to it
Add hydrogen atoms until every carbon has 4 bonds

How to name organic compounds
The names of organic compounds follow a set of specific rules based on the
Carbon chain length
The functional group
You can find the detailed rules for naming each family in their own revision notes:

Unlock more, it's free!
Was this revision note helpful?
