Structural Isomers (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Isomers
Isomers are molecules that have the same molecular formula but have different structural formulae
This means the atoms are connected in a different order
This means that they are different compounds and usually have different physical properties, e.g. different boiling points
Types of isomers
Isomers can come in different forms
The main types involve changes to:
The carbon chain
The position of a functional group
The overall homologous series
1. Chain isomers
These isomers have a different arrangement of the carbon chain
One is a straight chain, and the other is a branched chain
For example, isomers of butane, C4H10
Butane:
Is a straight chain of 4 carbon atoms
Methylpropane:
Has a straight chain of 3 carbon atoms
There is a carbon atom branched off the central carbon in the main chain
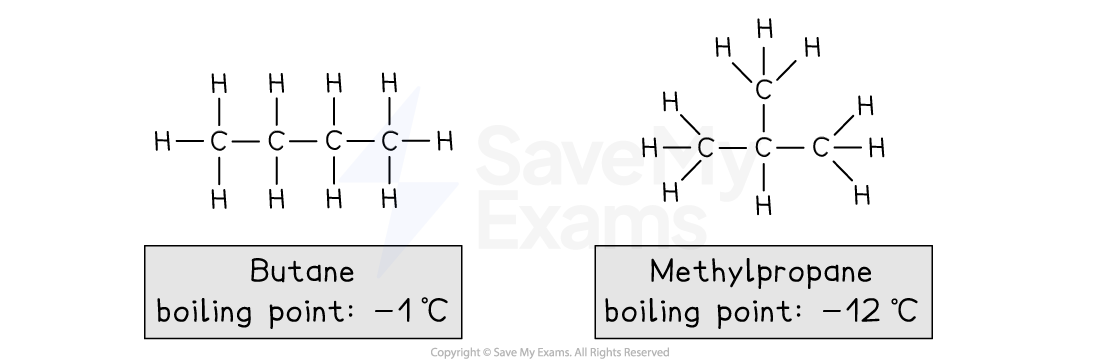
Chain isomers are possible for alkanes with 4 or more carbon atoms
Physical properties of isomers
Isomers usually have different physical properties, like boiling points
For alkanes, there is a specific trend related to branching.
For isomers with the same number of carbon atoms, increased branching leads to a lower boiling point.
For example, the isomers of C5H12:
Isomer | Structure | Boiling point (°C) |
|---|---|---|
pentane | straight chain | 36 |
2-methylbutane | branched | 28 |
2,2-dimethylpropane | highly branched | 9 |
As the amount of branching increases, the boiling point decreases.
Examiner Tips and Tricks
You are not expected to explain why branching lowers the boiling point at National 5
You just need to be able to identify and state this trend if you are given a table of data in an exam
2. Positional isomers
These isomers have the same carbon chain and the same functional group, but the functional group is attached to a different carbon atom
The functional group is described as being in a different position
For example, isomers of butene, C4H8
But-1-ene:
Has a chain of 4 carbon atoms
The C=C double bond starts at carbon 1
But-2-ene:
Has a chain of 4 carbon atoms
The C=C double bond starts at carbon 2
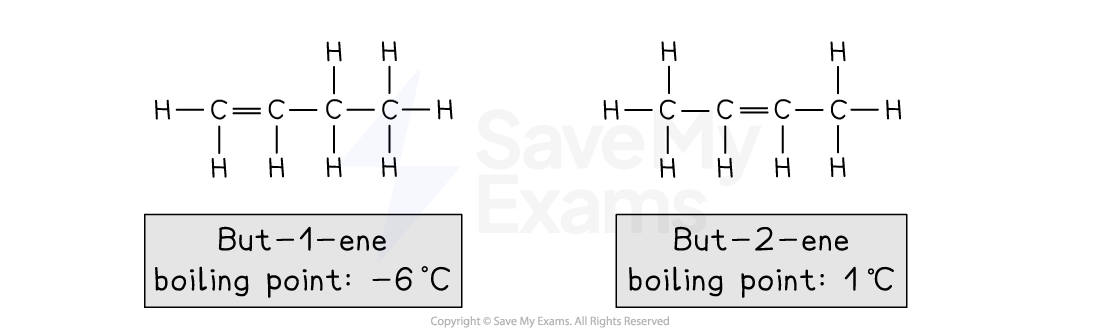
Positional isomers can occur in:
Alkenes with 4 or more carbon atoms
Alcohols with 3 or more carbon atoms
Positional isomers do not occur in carboxylic acids, as the carboxyl group is always at the end of the chain (on carbon 1)
3. Isomers in different homologous series
Isomers with the same molecular formula can belong to completely different families of compounds
This happens when the atoms are arranged to form different functional groups
This type of isomerism is sometimes called functional group isomerism
For example, isomers of propene, C3H6
Propene:
Is an alkene
Its functional group is the C=C double bond
Cyclopropane:
Is a cycloalkane
Its key structural feature is the ring of C-C single bonds
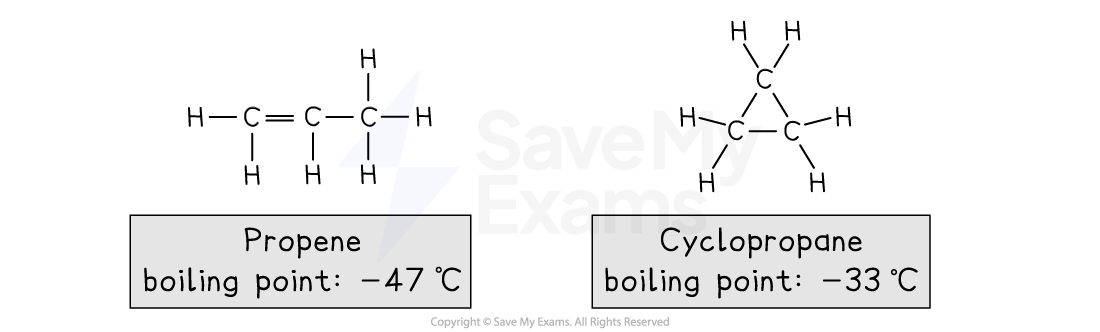
The different functional groups give these isomers different chemical properties
For example, propene will decolourise bromine solution, but cyclopropane will not
Examiner Tips and Tricks
Isomers in different homologous series can occur in many places
But for the SQA National 5 course, you need to be aware:
Alkenes and cycloalkanes
They have the same number of carbon atoms
They have the same general formula CnH2n
They have a different full structural formula
How to draw isomers
Drawing all the isomers for a given molecular formula can be challenging
You need to have a methodical and logical system that avoids drawing the same molecule twice
Drawing isomers of pentane, C5H12
Draw the straight-chain isomer
Steps:
Always start with the simplest structure
This is a straight chain of all the carbon atoms
Then, add the hydrogen atoms so that every carbon has 4 bonds
For C5H12:
Draw a 5-carbon chain
Add the 12 hydrogen atoms - remember the "Four Bond" rule

Shorten the main chain by one carbon
Steps:
Make the main chain one carbon shorter
Use that "leftover" carbon as a branch
For C5H12:
Draw a 4-carbon chain (a butane chain)
You have a 1-carbon branch (-CH3, a methyl group) to add
You can't add this branch to carbon 1 or carbon 4 (the ends)
This would just make a 5-carbon chain again
You can add it to carbon 2 or carbon 3
Both positions actually give you the same molecule, just flipped over
So, we'll add it to carbon 2
Add the hydrogens

Shorten the main chain again
Steps:
Make the chain even shorter
For C5H12:
Draw a 3-carbon chain (a propane chain)
You have two 1-carbon branches (two methyl groups) to add
The only place to put them without extending the chain is on the central (second) carbon
Add both methyl groups to carbon 2
Add the hydrogens

Pentane, C5H12, has three possible isomers
Worked Example
Draw the full structural formulae for four different isomers of butanol, C4H9OH.
Answer:
The formula C4H9OH indicates:
The longest main chain will be four carbon atoms
This means that we can have:
Positional isomers
Moving the alcohol functional group
Chain isomers
A straight chain of 4 carbon atoms
A chain of 3 carbon atoms with a 1-carbon branch on the central carbon

Positional isomers:
Start with the four carbon chain
The -OH group can be added to the first or second carbon atom
Then, add hydrogens so every carbon has 4 bonds

Chain isomers:
Start with the branched three carbon chain
The -OH group can be added to one of the end carbon atoms or the central carbon atom
Then, add hydrogens so every carbon has 4 bonds
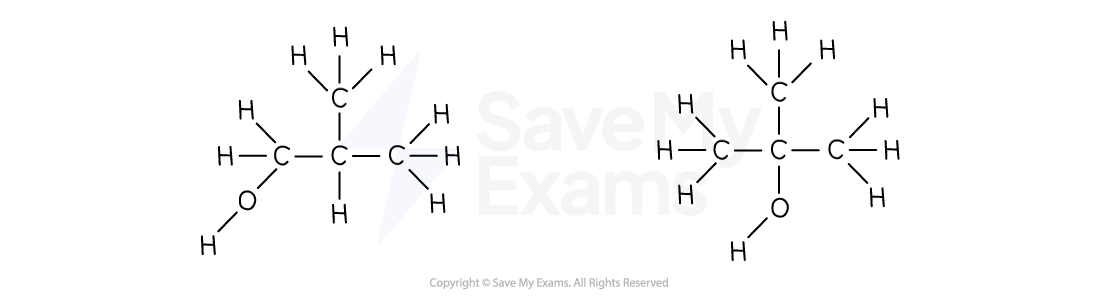
Examiner Tips and Tricks
To check if two structures are isomers, always count the atoms first:
Count the carbons and hydrogens in both molecules.
Are they the same?
If yes, check the structure.
Is the connection order different?
Is one branched while the other is straight?
If the answer to both questions is yes, they are isomers

Unlock more, it's free!
Was this revision note helpful?
