Systematic Carbon Chemistry (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Members of a homologous series
A homologous series is a family of organic compounds with a set of shared characteristics:
They have the same general formula
They have the same functional group, which gives them similar chemical properties
They show a gradual change (a trend) in their physical properties, such as boiling point
General formulae
The general formula tells you the composition of any member of a homologous series
You can use it to work out the molecular formula for any member of a homologous series, just by knowing the number of carbon atoms, n
General formulae of homologous series
Homologous series | General formula |
|---|---|
CnH2n+2 | |
CnH2n | |
CnH2n | |
CnH2n+1OH | |
CnH2n+1COOH |
For example:
Alkanes have the general formula CnH2n+2
n represents the number of carbon atoms
So, taking the number of carbon atoms in the alkane, doubling it and adding two gives the number of hydrogen atoms in the alkane
Worked Example
Questions
What is the formula of an alcohol that contains 5 carbon atoms?
What is the formula of an alkene that contains 10 carbon atoms?
Answers:
1. The formula of an alcohol containing 5 carbon atoms is:
General formula for an alcohol = CnH2n+1OH
Number of carbons = 5
Number of hydrogen atoms (excluding in the functional group) = 2 x 5 + 1 = 11
Formula = C5H11OH
2. The formula of an alkene that contains 10 carbon atoms is:
General formula for an alkene = CnH2n
Number of carbons = 10
Number of hydrogen atoms = 2 x 10 = 20
Formula = C10H20
Functional groups
A functional group is the specific group of atoms in a molecule that is responsible for its characteristic reactions
It is the most important part of the molecule, as it determines how the compound will react
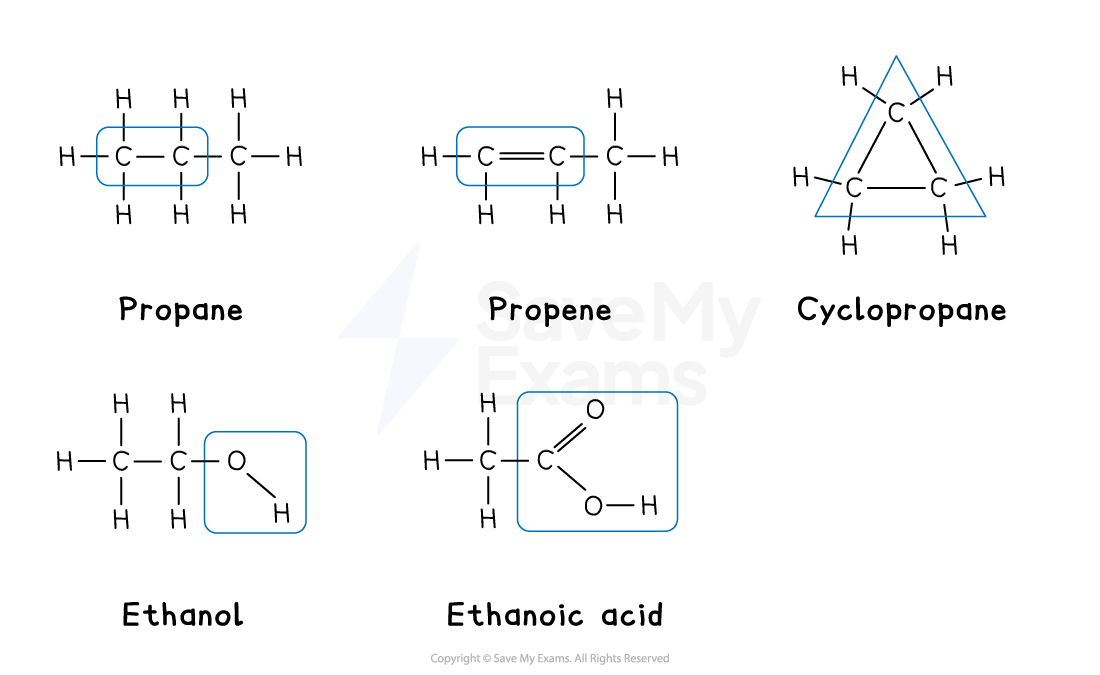
You need to be able to identify the functional group for each homologous series:
Homologous series and their functional groups
Homologous series | Functional group | Name |
|---|---|---|
Alkane | C-C (single bonds) | ends in -ane |
Cycloalkane | Ring of C-C single bonds | contains cyclo ends in -ane |
Alkene | C=C (double bond) | ends in -ene |
Alcohol | -OH (hydroxyl) | ends in -ol |
Carboxylic acid | -COOH (carboxyl) | ends in -anoic acid |
[GRAPHIC: Functional Group Examples]
Trends in physical properties
As you go up a homologous series, the melting and boiling points increase.
Explaining the trend
As the size of the molecule increases, the strength of the intermolecular forces increases
Intermolecular forces are the weak forces of attraction between the molecules
This means that more energy is needed to overcome these stronger forces
So, the melting and boiling points increase
Example: The alkanes
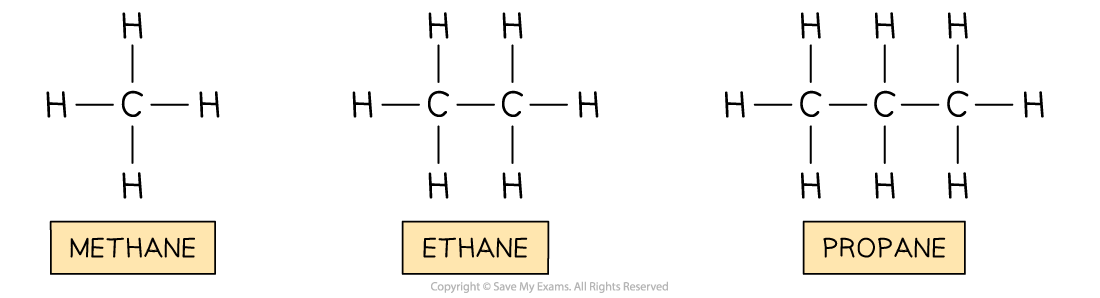
Alkane | Molecular formula | Boiling point (oC) |
|---|---|---|
Methane | CH4 | -162 |
Ethane | C2H6 | -89 |
Propane | C3H8 | -42 |
As the molecules get bigger, the boiling points increase from -162 oC to -42 oC
This same trend applies to other homologous series, such as alcohols and carboxylic acids

Unlock more, it's free!
Was this revision note helpful?
