Which of the following oxides, when shaken with water, would give an alkaline solution?
You may wish to use the data booklet to help you.
Calcium oxide
Nitrogen dioxide
Sulfur dioxide
Nickel oxide
Was this exam question helpful?
Exam code: X813 75
Which of the following oxides, when shaken with water, would give an alkaline solution?
You may wish to use the data booklet to help you.
Calcium oxide
Nitrogen dioxide
Sulfur dioxide
Nickel oxide
Choose your answer
Was this exam question helpful?
A student made some statements about the effect of adding water to a solution of hydrochloric acid.
Identify the correct statement.
The rate of reaction with magnesium will increase.
The concentration of H+ ions will increase.
The acid will be neutralised.
The pH will increase.
Choose your answer
Was this exam question helpful?
Which of the following compounds, when added to water, will change its pH?
You may wish to use the data booklet to help you.
Aluminium oxide
Calcium oxide
Copper(II) oxide
Zinc(II) oxide
Choose your answer
Was this exam question helpful?
25 cm3 of water was added to an aqueous solution of sodium hydroxide.
Compared to the initial sodium hydroxide solution, the final sodium hydroxide solution has
a lower number of moles of sodium hydroxide and a higher pH
the same number of moles of sodium hydroxide and a higher pH
the same number of moles of sodium hydroxide and a lower pH
a lower number of moles of sodium hydroxide and a lower pH.
Choose your answer
Was this exam question helpful?
Which of the following substances, when shaken with water, would cause the pH of water to increase?
You may wish to use the data booklet to help you.
Aluminium oxide
Barium oxide
Nitrogen oxide
Hydrogen oxide
Choose your answer
Was this exam question helpful?
Nickel carbonate, nickel hydroxide and nickel metal all react with dilute sulfuric acid.
Which of the following statements is true for all three reactions?
A gas is produced
Water is produced.
Nickel sulfate is produced.
A neutralisation reaction takes place.
Choose your answer
Was this exam question helpful?
Sodium carbonate can be used to neutralise hydrochloric acid.
(Na+ )2CO3 2− (aq) + 2H+ Cl− (aq) 2Na+ Cl− (aq) + H2O(ℓ) + CO2(g)
The correct equation omitting the spectator ions is
H+ (aq) + OH− (aq) H2O(ℓ)
2H+ (aq) + CO3 2− (aq) H2O(ℓ) + CO2(g)
2H+ (aq) + CO3 2− (g) H2O(ℓ) + CO2(g)
Na+ (aq) + Cl− (aq) Na+ Cl− (aq)
Choose your answer
Was this exam question helpful?
A titration was carried out to neutralise 0.002 mol of sulfuric acid solution, H2SO4.
2NaOH(aq) + H2SO4(aq) Na2SO4(aq) + 2H2O(ℓ)
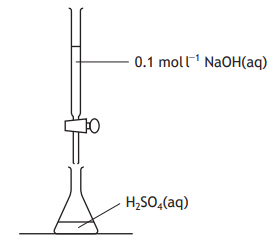
The number of moles of NaOH(aq) required to neutralise the acid is
0.05
0.004
0.002
0.001
Choose your answer
Was this exam question helpful?
Which of the following compounds is a salt?
Calcium oxide
Hydrogen nitrate
Sodium hydroxide
Potassium ethanoate
Choose your answer
Was this exam question helpful?
The pH of the solution formed when ammonia is bubbled into water is most likely to be:
3
5
7
9
Choose your answer
Was this exam question helpful?
Limewater can be made by dissolving calcium hydroxide in water.
Which of the following terms correctly describes calcium hydroxide?
Solute
Solvent
Solution
Insoluble
Choose your answer
Was this exam question helpful?
As an alkaline solution is diluted with water
the pH increases
the pH stays the same
the concentration of hydroxide ions increases
the concentration of hydroxide ions decreases.
Choose your answer
Was this exam question helpful?
Which of the following compounds is a base?
Sodium oxide
Calcium chloride
Potassium nitrate
Ammonium sulfate
Choose your answer
Was this exam question helpful?
Which of the following ions are spectator ions in the reaction?
Ag+ and NO3 −
Na+ and NO3 −
Ag+ and Br−
Na+ and Br−
Choose your answer
Was this exam question helpful?
An alkaline solution contains
only hydroxide ions
more hydroxide ions than hydrogen ions
more hydrogen ions than hydroxide ions
equal numbers of hydrogen ions and hydroxide ions.
Choose your answer
Was this exam question helpful?
A student made some statements about the effect of adding water to an acidic solution.
Identify the correct statement.
The pH of the solution will remain the same.
The pH of the solution will decrease.
The hydrogen ion concentration will decrease.
The hydrogen ion concentration will increase.
Choose your answer
Was this exam question helpful?