Oganesson is a newly discovered element with a predicted electron arrangement of 2,8,18,32,32,18,8
In which group of the periodic table should oganesson be placed?
0
1
2
7
Was this exam question helpful?
Exam code: X813 75
Oganesson is a newly discovered element with a predicted electron arrangement of 2,8,18,32,32,18,8
In which group of the periodic table should oganesson be placed?
0
1
2
7
Choose your answer
Was this exam question helpful?
Which line in the table shows the correct number of protons and electrons for the atom,?
Protons | Electrons | |
A | 56 | 56 |
B | 56 | 26 |
C | 26 | 26 |
D | 26 | 56 |
Choose your answer
Was this exam question helpful?
Which bonding and structure is never found in elements?
Covalent molecular
Ionic lattice
Metallic lattice
Covalent network
Choose your answer
Was this exam question helpful?
Which of the following compounds would conduct electricity at 600 °C?
You may wish to use the data booklet to help you.
Silicon dioxide
Lithium bromide
Ammonia
Barium chloride
Choose your answer
Was this exam question helpful?
Which two lines in the table show particles that are isotopes of the same element?
Particle | Number of protons | Number of neutrons | Number of electrons |
W | 11 | 12 | 10 |
X | 11 | 12 | 11 |
Y | 12 | 12 | 12 |
Z | 12 | 14 | 12 |
W and X
X and Z
W and Y
Y and Z
Choose your answer
Was this exam question helpful?
An atom of tin has 74 neutrons.
Which of the following is the mass number of this atom?
You may wish to use the data booklet to help you.
50
74
118.5
124
Choose your answer
Was this exam question helpful?
Lead forms many compounds with non-metal elements.
At room temperature tetraethylplumbane, Pb(C2H5)4, is a liquid that does not conduct electricity and is insoluble in water.
The structure and bonding in tetraethylplumbane is
covalent molecular
covalent network
ionic lattice
metallic lattice.
Choose your answer
Was this exam question helpful?
Which line in the table correctly describes a proton?
Mass | Charge | Location | |
A | 1 | +1 | inside the nucleus |
B | 0 | −1 | outside the nucleus |
C | 1 | 0 | outside the nucleus |
D | 0 | +1 | inside the nucleus |
Choose your answer
Was this exam question helpful?
Which of the following compounds forms molecules with an angular structure?
CCl4
NCl3
SCl2
FCl
Choose your answer
Was this exam question helpful?
Which of the following diagrams could be used to represent the structure of lithium fluoride?
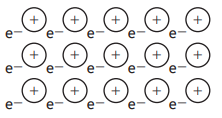
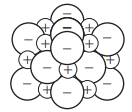

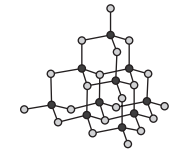
Choose your answer
Was this exam question helpful?
Which of the following is the atomic number of a metal?
1
33
45
86
Choose your answer
Was this exam question helpful?
An atom is neutral because:
the number of protons equals the number of neutrons
the number of electrons equals the number of protons
the number of electrons equals the number of protons plus neutrons
the number of neutrons equals the number of electrons plus protons
Choose your answer
Was this exam question helpful?
When liquid water changes to steam:
weak forces of attraction between the water molecules are broken
strong forces of attraction between the water molecules are broken
weak forces of attraction between the atoms in the water molecules are broken
strong forces of attraction between the atoms in the water molecules are broken.
Choose your answer
Was this exam question helpful?
Which of the following structures would be described as angular?
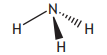
Choose your answer
Was this exam question helpful?
Electronegativity is a measure of the attraction a nucleus has for the shared pair of electrons in a covalent bond.
When two nuclei that have different electronegativity values are bonded together, the bond formed is described as ‘polar covalent’.
The bigger the difference in the electronegativity values the more polar the bond.
The table contains electronegativity values for some atoms.
Atom | Electronegativity value |
H | 2.2 |
C | 2.6 |
N | 3.0 |
O | 3.4 |
Which of the following bonds would be the most polar?
O−H
N−H
C−H
C−O
Choose your answer
Was this exam question helpful?
Tennessine is a newly discovered element with a predicted electron arrangement of 2,8,18,32,32,18,7.
In which group of the periodic table should Tennessine be placed?
1
2
7
8
Choose your answer
Was this exam question helpful?
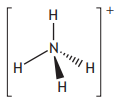
Which of the following is a positively charged ion?
Protons | Neutrons | Electrons | |
A | 9 | 10 | 10 |
B | 10 | 9 | 10 |
C | 11 | 12 | 11 |
D | 12 | 13 | 10 |
Choose your answer
Was this exam question helpful?
To turn a gas into a liquid it must be cooled below a temperature known as its critical temperature.
Gas | Formula | Relative formula mass | Critical temperature (°C) |
hydrogen | H2 | 2 | −240 |
helium | He | 4 | −268 |
ammonia | NH3 | 17 | 133 |
oxygen | O2 | 32 | −119 |
carbon dioxide | CO2 | 44 | 31 |
Identify the true statement based on the information in this table.
Carbon dioxide can be a liquid at 40 °C
Compounds have higher critical temperatures than elements
Critical temperature increases as relative formula mass increases
Diatomic elements have lower critical temperatures than Noble gases
Choose your answer
Was this exam question helpful?
A molecule of phosphorus trifluoride is shown.
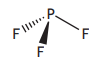
Which term can be used to describe the shape of a phosphorus trifluoride molecule?
Linear
Angular
Tetrahedral
Trigonal pyramidal
Choose your answer
Was this exam question helpful?
In which of the following compounds do the ions have the same electron arrangement?
You may wish to use the data booklet to help you.
Na2O
LiF
KBr
MgCl2
Choose your answer
Was this exam question helpful?
Several conductivity experiments were carried out using the apparatus below.
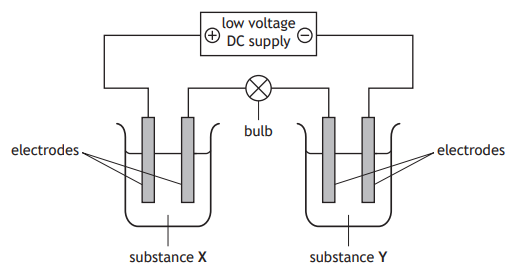
Identify the experiment in which the bulb would light.
Substance X | Substance Y | |
A | solid copper sulfate | liquid mercury |
B | copper chloride solution | molten sodium chloride |
C | solid potassium nitrate | nickel bromide solution |
D | sodium chloride solution | liquid hexane |
Choose your answer
Was this exam question helpful?
Which line in the table identifies the correct location of a proton and an electron in an atom?
Proton | Electron | |
A | inside the nucleus | inside the nucleus |
B | inside the nucleus | outside the nucleus |
C | outside the nucleus | outside the nucleus |
D | outside the nucleus | inside the nucleus |
Choose your answer
Was this exam question helpful?
Which of the following elements does not exist as diatomic molecules?
Oxygen
Helium
Bromine
Hydrogen
Choose your answer
Was this exam question helpful?
The shapes of some molecules are shown below.
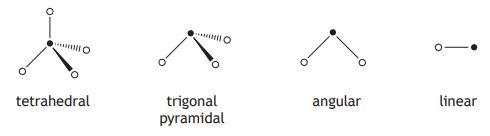
The shape of a molecule of hydrogen bromide is likely to be
tetrahedral
trigonal pyramidal
angular
linear.
Choose your answer
Was this exam question helpful?
Which of the following elements forms an ion with a single positive charge and an electron arrangement of 2,8?
You may wish to use the data booklet to help you.
Sodium
Magnesium
Fluorine
Neon
Choose your answer
Was this exam question helpful?
Which line in the table shows the properties of a covalent network compound?
Melting point (°C) | Boiling point (°C) | Conducts electricity | ||
Solid | Liquid | |||
A | -127 | -100 | no | no |
B | 795 | 1410 | no | yes |
C | 30 | 2204 | yes | yes |
D | 2700 | 3350 | no | no |
Choose your answer
Was this exam question helpful?