1
1 mark
Polymethylmethacrylate is a polymer used in the manufacture of aircraft windows. A section of the polymer chain is drawn below.
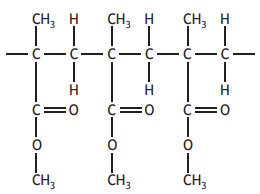
The monomer used to make this polymer is:
Was this exam question helpful?
Exam code: X813 75
Polymethylmethacrylate is a polymer used in the manufacture of aircraft windows. A section of the polymer chain is drawn below.

The monomer used to make this polymer is:
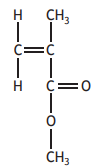
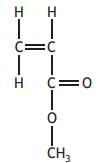
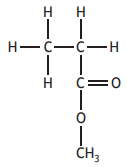
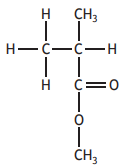
Choose your answer
Was this exam question helpful?
A co-polymer is formed when two different monomers polymerise.
Part of the structure of a co-polymer, showing three monomer units, is given below.
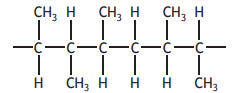
One of the monomers used is propene.
Identify the other monomer.
Pent-2-ene
Pent-1-ene
But-2-ene
But-1-ene
Choose your answer
Was this exam question helpful?