Hardest AP Chemistry Questions & How to Answer Them
Written by: Richard Boole
Reviewed by: Philippa Platt
Last updated
Contents
Preparing for the AP Chemistry exam means facing some questions designed to stretch even the top students. These questions require more than just memorization. They test your ability to:
Think critically
Apply principles in new contexts
Build a logical argument.
The most difficult questions separate the 5s from the 4s. But is the subject's reputation for being difficult really deserved? For a full statistical breakdown, check out our in-depth guide: Is AP Chemistry Hard?
A useful analogy is to think of it like a Rubik's Cube. Simple AP questions might ask you to solve one side (a single concept). But, the hardest ones require you to:
Apply multiple, interconnected algorithms (concepts and mathematical routines)
In a specific sequence
While knowing how each move affects the entire structure.
Drawing on a deep analysis of official College Board scoring data and Chief Reader Reports, this guide breaks down the hardest AP questions and shows you exactly how to solve them.
Key Takeaways
The hardest AP Chemistry questions test skills, not just content. They focus on:
Synthesizing knowledge from different units (e.g., Electrochemistry and Thermodynamics).
Navigating multi-step calculations where the path isn't immediately obvious.
Justifying claims with precise evidence and reasoning.
Success comes from a systematic approach:
Break down multi-part FRQs
Identify key data
Showing your work is crucial for securing "method points," even if your final answer slips up.
What Makes an AP Chemistry Question Hard?
To understand what makes a question hard, you need to know what the College Board is testing. Your final AP score is determined by your mastery of both course content and specific Science Practices.
The hardest questions are deliberately designed to be heavy on higher-order Science Practices, specifically:
Practice 5 (Mathematical Routines):
Can you create a logical pathway through a complex calculation where the steps are not explicitly prompted?
Practice 6 (Argumentation):
Can you make a scientific claim and support it with specific evidence?
Hardest AP Chemistry Topics
Analysis of official exam data shows that certain topics consistently challenge students. While any of the AP Chemistry Units can be made difficult, these areas are frequent sources of the trickiest questions on the exam.
Equilibrium and Le Châtelier’s Principle
Equilibrium is a central concept in AP Chemistry and a major source of difficult questions. Students often struggle with:
Complex Kp or Kc calculations
Misinterpreting titration graphs
Applying Le Châtelier’s Principle, especially in unfamiliar contexts like dilution.
Electrochemistry and Redox Reactions
This topic demands a high level of procedural accuracy. Common struggles include:
Correctly identifying the anode and cathode in a galvanic cell
Constructing and balancing complex half-reactions
Correctly calculating standard cell potentials (E°cell).
Thermodynamics and Gibbs Free Energy
Thermodynamics questions test deep conceptual understanding. Students often find it difficult to connect the signs of enthalpy (ΔH) and entropy (ΔS) to the overall thermodynamic favorability (spontaneity) of a reaction using the Gibbs free energy equation (ΔG = ΔH - TΔS).
Stoichiometry in Limiting Reagent Problems
While stoichiometry is a foundational skill, AP-level questions often involve multiple steps, sometimes starting with experimental data and requiring you to work backwards or handle excess reactants. Unit conversions and mole ratios must be handled perfectly.
Acid-Base Equilibria and Titration Curves
This is a major source of challenging FRQs. Difficulties include:
Calculating the pH of buffer solutions.
Interpreting the key points on a titration curve (like the half-equivalence point where pH = pKa).
Explaining how a buffer works at the particle level.
Types of Tricky AP Chemistry Questions
Beyond specific topics, certain question formats are designed to be tricky. Mastering these formats is key to achieving a top score.
Conceptual MCQs with Distractors
The hardest Multiple-Choice Questions (MCQs) are not calculation-based. They are conceptual questions with cleverly designed "distractors", that are wrong answers that look plausible if you have a superficial understanding of the topic. These questions test whether you can go beyond memorized rules and apply the underlying principles correctly.
Multi-Part FRQs
Long Free-Response Questions (FRQs) are challenging because they require stamina and an ability to connect ideas. Often, the parts of a single FRQ are linked; an answer from part (a) may be needed for part (c). These questions test your ability to organize your thoughts and manage your time effectively over 10 points.
Particle Diagram Questions
Questions that use particle diagrams to represent atoms, ions, and molecules are notoriously difficult. Students often struggle to translate between the macroscopic world of numbers and graphs and the microscopic world of particles. You must pay close attention to the orientation, spacing, and relative number of particles.
Worked Examples of the Hardest AP Chemistry Questions
These examples, based on real AP exam questions identified as having low success rates, illustrate the toughest question types and how to build a top-scoring answer.
Example 1 – Kinetics MCQ (Graphical Data)
This question tests your ability to interpret graphical data to determine reaction order.
The Question:
The following fourth-order reaction was studied at a constant temperature:
5Br- (aq) + BrO3- (aq) + 6H+ (aq) → 3Br2 (aq) + 3H2O (l)
Data was collected for the rate of the reaction as follows:
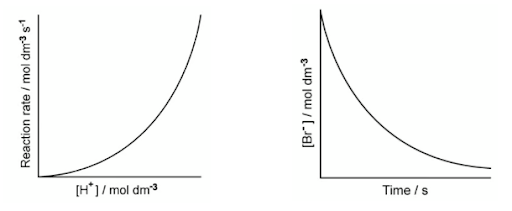
Which rate expression is consistent with the data?
(A) Rate = k[Br-][BrO3-][H+]
(B) Rate = k[Br-]2[H+]2
(C) Rate = k[Br-][BrO3-][H+]2
(D) Rate = k[Br-]2[BrO3-][H+]
[Source: Save My Exams, Reaction Mechanisms topic, AP Chemistry Hard Question 2]
Why it's tricky:
Official AP analysis often shows low success rates on kinetics questions that rely on graphs rather than tables. Only 14% of students correctly answered a similar MCQ.
The difficulty lies in distinguishing between "Rate vs. Concentration" graphs and "Concentration vs. Time" graphs, as they follow different rules.
Worked Solution:
Step 1: Analyze the H+ graph (Rate vs. Concentration).
The graph plots Rate vs. [H+].
A plot of Rate vs. [Reactant] is linear only if the reaction is first order with respect to that reactant.
Here, the graph is an upward curve.
This shows the rate increasing
This indicates that it is not first order.
So, the reaction is second order with respect to H+.
Elimination: This eliminates option (A) and (D), which are first order for H+.
Step 2: Analyze the Br- graph (Concentration vs. Time).
The graph plots [Br-] vs. Time.
The shape of this curve determines the order.
A straight line with a negative slope would mean zero order.
A curve indicates it is either first or second order.
To distinguish between first and second order, you must look at the half-life (t1/2).
In this graph, the time it takes for the concentration to halve is constant.
It takes the same time to go from 1.0M to 0.5M as it does from 0.5M to 0.25M.
A constant half-life is the hallmark of a first-order reaction.
Elimination: This eliminates option (B), which is second order for Br−.
Step 3: Deduce the final rate law.
We've determined the order is 1 for [Br-] and 2 for [H+].
The question also states the overall reaction is fourth-order.
Total Order = Order(Br-) + Order(BrO3-) + Order(H+)
4 = 1 + Order(BrO3-) + 2
This means the order with respect to BrO3- must be 1.
The correct rate law is therefore Rate = k[Br-][BrO3-][H+]2, which is option (C).
Common Pitfalls:
Confusing the axes
Treating a "Rate vs. Conc" graph like a "Conc vs. Time" graph.
Always check the labels first.
Ignoring the Half-Life
Seeing a curve on a "Conc vs. Time" graph and guessing.
You must check if the half-life is constant to confirm it is first order.
How to succeed:
Know the shapes:
Memorize the characteristic shapes of 0, 1st, and 2nd order graphs for both Rate and Concentration axes.
Check the slope:
On a Concentration vs. Time graph, a straight line is zero order.
On a Rate vs. Concentration graph, a straight line is first order.
Example 2 – Solutions & intermolecular forces MCQ (Chromatography)
This question tests your ability to model the competing intermolecular forces between a solute, a stationary phase, and a mobile phase.
The Question:
A thin-layer chromatography (TLC) experiment was conducted using a silica gel-coated TLC plate (a polar material) and a solvent mixture of 95% dichloromethane (DCM) and 5% methanol. The sample mixture contained two polar food additives.
The relative polarities of the solvents used in this and similar experiments are:
Chloroform 0.26
DCM 0.31
Methanol 0.76
Based on this information, which change to the solvent mixture would likely increase the Rf values of the food additives in a subsequent experiment?
(A) Decreasing the percentage of methanol.
(B) Increasing the percentage of methanol.
(C) Replacing DCM with chloroform (relative polarity 0.26).
(D) Replacing methanol with chloroform.
[Source: Save My Exams, Solutions & Mixtures topic, AP Chemistry Hard Question 3]
Why it's tricky:
Questions involving chromatography are consistently difficult. Official analysis of a similar 2019 question showed a student success rate of only 25%. The difficulty lies in correctly relating three different interactions simultaneously:
The analyte with the stationary phase.
The analyte with the mobile phase.
The mobile phase with the stationary phase.
Worked Solution:
Step 1: Understand the goal.
We want to increase the Rf value. This means we want the spots (analytes) to travel further up the plate.
Step 2: Analyze the forces.
Stationary Phase: Silica gel is polar.
Analytes: The food additives are polar.
This means they will "stick" to the polar silica gel via dipole-dipole interactions or hydrogen bonding.
Mobile Phase: The solvent mixture is the mobile phase.
Its job is to carry the analytes up the plate.
Step 3: Determine how to move the spots.
To make the spots move further, we need the mobile phase (solvent) to compete more effectively with the stationary phase.
If we make the solvent more polar, it will dissolve the polar analytes better and pull them up the plate, overcoming their attraction to the silica gel.
Step 4: Evaluate the options.
We need to make the solvent more polar.
Methanol (0.76) is the most polar component. DCM (0.31) is less polar.
(A) Decrease methanol:
Makes solvent less polar.
Spots move less.
Rf decreases.
(B) Increase methanol:
Makes solvent more polar.
Spots move further.
Rf increases.
This is the correct answer.
(C) & (D) Replace with chloroform:
Chloroform (0.26) is even less polar than DCM.
This makes the solvent less polar.
Spots move less.
Rf decreases.
Common Pitfalls:
Ignoring polarity values:
Students often guess based on the names of the chemicals rather than using the data provided in the table.
Reversing Rf:
Confusing increasing Rf (moving further) with decreasing Rf (sticking to the start line).
How to succeed:
Like dissolves Like:
Remember that for a spot to move, it must "like" the mobile phase more than the stationary phase.
Check the Polarity:
Always identify which phase is polar and which is nonpolar before reading the answers.
Example 3 – Weak Acid Equilibrium FRQ (Dilution Effects)
This question tests your conceptual understanding of Le Châtelier’s Principle and reaction quotients in the context of weak acid ionization. It focuses on the weak acid equilibrium of hydrofluoric acid (HF). It asks students to predict and justify the effect on the percent ionization of HF when the solution is diluted by adding water:
The Question:
(c) If 50.0 mL of distilled water is added to 50.0 mL of 0.035 M HF (aq), will the percent ionization of HF (aq) in the solution increase, decrease, or remain the same? Justify your answer with an explanation or calculation.
[Source: Based on 2018 AP Chemistry FRQ, Q5]
Why it's tricky:
This overall question had a mean score of just 1.32 out of 4 (33%).
The Chief Reader Report noted that students consistently struggled to provide a correct justification. Many misused Le Châtelier's principle, incorrectly focusing on how dilution "shifts the equilibrium towards the side with more moles of solute," which is reasoning for pressure changes in gas equilibria, not for aqueous dilution.
Worked Solution:
Prediction: The percent ionization of HF will increase.
Justification (The "Q vs K" Method):
The equilibrium (given in the overall question) is:
HF (aq) + H2O (l) ⇌ H3O+ (aq) + F- (aq)
Step 1: Write the expression.
Ka = [H3O+][F-] / [HF]
Step 2: Analyze the dilution.
When water is added (dilution), the volume doubles.
This means that the concentration of every aqueous species ([HF], [H3O+], and [F-]) is halved.
Step 3: Calculate Q.
Since there are two species in the numerator and only one in the denominator, the value of the numerator decreases more than the denominator.
Mathematically:
Q = [0.5][0.5] / [0.5] = 0.5 Ka
This causes Q to become less than Ka.
Step 4:Conclude.
Since Q < Ka, the reaction must shift to the right (forward) to re-establish equilibrium.
A shift right produces more ions, increasing the percent ionization.
Common Pitfalls:
Vague Le Châtelier's arguments:
Stating "The reaction shifts to the side with more water" is not a valid chemical justification.
Confusing Moles vs Molarity:
Arguing that the number of moles hasn't changed, so equilibrium shouldn't shift (incorrect because equilibrium depends on concentration).
How to succeed:
Use the Le Châtelier’s Principle & Reaction Quotient (Q) method:
This is the "gold standard" justification for almost any equilibrium shift question. It is irrefutable evidence.
Example 4 – Multi-Concept FRQ (Bond Enthalpy)
This question tests your mastery of thermodynamics by requiring you to work backward from an enthalpy change to determine a specific bond energy.
The Question:
The compound BrCl can decompose into Br2 and Cl2, as represented by the balanced chemical equation below.
2BrCl (g) ⇌ Br2 (g) + Cl2 (g)
ΔHo = 1.6 kJ/molrxn
Calculate the bond energy of the Br-Cl bond, in kJ/mol, using ΔHo for the reaction and the information in the following table.
Bond | Bond Energy (kJ/mol) |
Br–Br | 193 |
Cl–Cl | 243 |
Br–Cl | ? |
[Source: 2019 AP Chemistry FRQ, Question 2(g)]
Why it's tricky:
This question flips the standard script. Usually, you are given all the bond energies and asked to find
ΔH. Here, you are given ΔH and must work backward to find a specific bond energy. This exposes two common weaknesses:
Formula confusion
Students often revert to "Products minus Reactants" (used for formation enthalpies) rather than "Broken minus Formed" (used for bond enthalpies).
Stoichiometry errors
Students frequently forget that the balanced equation has a coefficient of 2 in front of BrCl, meaning two moles of bonds are broken.
Worked Solution:
Step 1: Write the correct formula for Bond Enthalpy.
Unlike other thermodynamic calculations, bond enthalpy is calculated based on bond breaking (endothermic, positive) and bond forming (exothermic, negative).
ΔHo = Σ(bonds broken) − Σ(bonds formed)
Step 2: Identify bonds broken and formed.
Broken (Reactants): We are breaking 2 moles of Br–Cl bonds. Let x be the bond energy of Br–Cl.
bonds broken = 2x
Formed (Products): We are forming 1 mole of Br–Br bonds and 1 mole of Cl–Cl bonds.
bonds formed = (1 × 193) + (1 × 243) = 436 kJ
Step 3: Set up the algebraic equation.
Substitute the known ΔHo (1.6 kJ) and the expressions from Step 2 into the formula:
1.6 = (2x) − (436)
Step 4: Solve for x.
1.6 + 436 = 2x
437.6 = 2x
x = 218.8
Final Answer:
The bond energy of the Br-Cl bond is 219 kJ/mol (rounded to 3 significant figures).
Common Pitfalls:
Bond energies
Bond energies are average values, so small differences from data tables are expected.
The "Products - Reactants" trap:
If you use the ΔHf formula logic here, you will get the wrong sign and wrong value.
The coefficient trap:
Forgetting the "2" in front of the Br-Cl bond energy is the most common calculation error.
How to succeed:
Draw the molecules:
Sketch the Lewis structures to ensure you count every bond being broken and formed.
Check the Sign:
Bond breaking is always positive; bond forming is always negative.
Strategies to Improve Your Performance on Tough Questions
Master the AP Chemistry Equation Sheet
The equation sheet is your best friend in the exam, but only if you know how to use it.
Know What's On It
Familiarize yourself with every equation provided.
Know what each variable stands for and in what context to use it.
Know What's NOT On It
Just as importantly, know what isn't there.
You won't find the combined gas law, the dilution equation (M1V1=M2V2) or the specific rules for "Products - Reactants" vs "Broken - Formed".
You are expected to know these or derive them.
Use the Scoring Guidelines to Your Advantage
The best way to understand how to get points is to think like a grader.
Practice with FRQs and Rubrics:
After attempting a practice FRQ, don't just check the answer.
Study the official Scoring Guidelines from the College Board website.
See how points are awarded for specific phrases, calculations, and justifications.
Look for "1 Point For...":
Notice how rubrics break down a question into discrete points.
This trains you to structure your answers to hit each required point clearly and concisely.
Don't leave it blank:
In multi-step calculations, you can often earn points in Part (c) using an incorrect answer from Part (b), provided your logic in Part (c) is correct.
This is called "consistency" scoring.
Practice Under Exam Conditions
AP Chemistry is a marathon. Building mental stamina is as important as knowing the content.
Timed Practice:
Do full FRQ sections under timed conditions (approx. 23 minutes for a long question, 9 for a short one).
This helps you internalize the pacing you'll need on exam day.
Identify Weaknesses:
Timed practice quickly reveals where you slow down or make careless errors under pressure.
Use this information to focus your revision on your weakest areas.
For example, if you stare at a Kinetics graph for 3 minutes, that is a specific skill gap you need to target.
Frequently Asked Questions
What is the hardest topic in AP Chemistry?
While it varies for each student, exam data consistently shows that Equilibrium (including acid-base and solubility) and Electrochemistry are the topics where students lose the most points. This is because these questions often require multi-step calculations and deep conceptual justification.
How can I improve on AP Chemistry FRQs?
Practice, practice, practice. But, do it smartly. Use past FRQs from the College Board, and after each one, score your own response using the official Scoring Guidelines. This is the single best way to learn how to structure your answers to earn maximum points.
What score do I need on the FRQs to get a 5?
The FRQ section and the MCQ section are each worth 50% of your total score. There is no set "passing score" for the FRQs. To get a 5, you need a high overall composite score. Top-scoring students typically demonstrate a strong command of the FRQs, often earning a majority of the available points across all seven questions.
Do I need to memorise all the equations?
No. A comprehensive equation sheet is provided with the exam. Your job is not to memorize the equations but to understand what they mean, what each variable represents, and when and how to apply them correctly.
Can I improve my score by focusing just on hard topics?
While it's important to strengthen your weaknesses, a top score requires a strong foundation in all nine units of the course. The hardest questions are often synoptic, meaning they combine ideas from "easy" topics (like stoichiometry) with "hard" topics (like equilibrium). A balanced approach to revision is the most effective strategy.
Final Thoughts
The hardest AP Chemistry questions are designed to be challenging, but they are never impossible. They reward students who have moved beyond memorization and have developed a true conceptual understanding of chemistry.
Approaching these questions strategically makes them far more manageable:
Break them down.
Apply systematic methods.
Practice with real past papers and examiner reports.
Don't view difficult questions as a barrier. See them as an opportunity to demonstrate your mastery of the subject. With the techniques in this guide, you'll be ready to tackle them with confidence.
Mastering these questions is a key part of securing a top grade. For a complete roadmap covering revision planning and effective study techniques, read our comprehensive guide on: How to Score a 5 in AP Chemistry.
Now that you're equipped with the expert strategies to deconstruct and master AP Chemistry's toughest questions, the final step is to put them into practice. Our resources are tailor-made to help you apply these new skills and build exam-winning habits:
Study guides
Solidify your understanding of the core principles with our concise, syllabus-aligned study guides before you tackle the hard questions.
Topic questions & model answers
Practise the specific types of problems we've covered with our exam-style topic questions. Each one is created by an expert and comes with a detailed answer, showing you what a top-grade response looks like.
Past papers:
Simulate real exam conditions and perfect your timing with our complete library of past exams, scoring guidelines and other official resources.
Stop worrying about the hardest questions and start conquering them today.
References
The College Board - AP Chemistry (opens in a new tab)
Sign up for articles sent directly to your inbox
Receive news, articles and guides directly from our team of experts.

Share this article
 written revision resources that improve your
written revision resources that improve your