Hardest IB Chemistry Questions & How to Answer Them
Written by: Richard Boole
Reviewed by: Philippa Platt
Last updated
Contents
- 1. Key Takeaways
- 2. What Makes a Question Hard in IB Chemistry?
- 3. Most Difficult IB Chemistry Topics
- 4. HL vs SL Difficulty Differences
- 5. Difficult IB Chemistry Question Types
- 6. Worked Examples of the Hardest IB Chemistry Questions
- 7. How to Improve at High-Level IB Chemistry Questions
- 8. Frequently Asked Questions
- 9. Final Thoughts
- 10. References
If you are aiming for a 6 or 7, you cannot rely on rote memorization. Is IB Chemistry hard? It certainly can be, primarily because the new 2025 syllabus has shifted the focus significantly toward conceptual application. The hardest questions in the new Paper 1 and Paper 2 now test your ability to link "Structure" with "Reactivity" in unfamiliar contexts.
To master what is IB Chemistry at the highest level, you need to understand why certain questions are difficult. This guide breaks down the toughest questions, explains the new assessment format, and provides model answers to help you approach exams with clarity and confidence.
Key Takeaways
The "New" Paper 1 Trap
Paper 1 is no longer just simple multiple-choice.
It is split into Booklet A (MCQ) and Booklet B (Data-based).
The hardest data analysis questions (formerly Paper 3) are now found here.
Calculators change everything
Since calculators are now permitted in Paper 1, examiners are setting "harder" MCQs involving complex, multi-step stoichiometry and thermodynamics.
Synoptic thinking
The most difficult questions force you to merge syllabus areas.
For example, using periodic trends (Structure) to predict reaction feasibility (Reactivity).
Precision is key
High-mark questions often rely on specific technical skills, such as:
Drawing 3D transition states in organic mechanisms
Using precise terminology for d-orbital splitting.
What Makes a Question Hard in IB Chemistry?
A question isn't "hard" simply because it asks for a fact you haven't memorized. In the IB Diploma Programme, difficulty is engineered through specific structural choices.
1. Synoptic links (multi-topic integration)
The syllabus is no longer a list of isolated topics; it is a matrix of Structure and Reactivity.
A difficult question might ask you to predict the mechanism of a reaction (Reactivity 3.4) based solely on the hybridisation and steric hindrance of the molecule (Structure 2.2).
If you study these concepts in isolation, you will struggle to make the connections.
2. Unfamiliar contexts
The IB loves to present data on compounds you have never seen before.
They might give you a complex pharmaceutical drug or a rare mineral.
The difficulty lies in panic control: you aren't supposed to know the compound, but you are supposed to recognise the functional group or the stoichiometric ratio buried in the data.
3. The "command term" trap
Misinterpreting the command term is the most common non-chemistry error.
"Describe" means to give a detailed account, while "Explain" requires a cause and effect.
If a difficult question asks you to "Evaluate" experimental data, simply describing the graph will cap your marks at 50%.
Most Difficult IB Chemistry Topics
While every student has different strengths, examiner reports consistently highlight specific areas that yield the lowest average scores. You can review the full IB Chemistry Topic list here.
Thermochemistry & Energetics
Specifically, Hess’s Law cycles and Born-Haber cycles.
The difficulty lies in the "bookkeeping" of signs (+/-) and state changes (gas to liquid).
Organic Mechanisms (HL)
Drawing the transition states for SN2 reactions or understanding the stereochemical implications of nucleophilic substitution often separates the 7s from the 6s.
Transition Metals (HL)
This topic requires precise explanations involving d-orbital splitting and light absorption.
Vague answers regarding "electron jumping" often score zero.
Acids and Bases (HL & SL)
Specifically, distinguishing between strong/weak vs. concentrated/dilute, and the complex logic required for buffer solution calculations.
HL vs SL Difficulty Differences
The gap between Standard Level and Higher Level is significant.
Quantitative Depth
In Acid-Base chemistry, SL students calculate pH for strong acids.
HL students must handle buffer solutions, pKa calculations, and the Henderson-Hasselbalch equation.
Abstract Concepts
SL covers the basics of bonding.
HL introduces hybridization (sp, sp2, sp3) and formal charge, which requires a much stronger grasp of 3D spatial reasoning.
Synthesis
HL questions require more synthesis.
You might need to apply kinetics knowledge to equilibrium problems or link organic mechanisms to energetics considerations.
Difficult IB Chemistry Question Types
Beyond specific topics, the format of the question often dictates its difficulty. For a breakdown of the papers, read our guide on how many IB Chemistry papers there are.
Data-Based Questions (Paper 1B)
Under the 2025 syllabus, these questions are now found in Paper 1, Booklet B, and Paper 2.
These questions provide a table, graph, or set of experimental results that may contradict "standard" textbook trends. The challenge here is anomaly detection and trend analysis. Students often ignore the data provided and try to answer based on what they remember from the textbook rather than what is in front of them.
Multi-Step Calculations
Usually found in Stoichiometry, Equilibrium, or Energetics. The trap here is losing track of units or "rounding errors" in early steps. With calculators now allowed in Paper 1, expect these questions to appear in multiple-choice formats as well, where one small error leads to a plausible (but incorrect) distractor answer.
Experimental Design & Practical Scenarios
These questions ask you to evaluate a student's hypothesis or improve a method. The trap is giving generic answers like "repeat the experiment." To succeed, you must be specific: "Use a gas syringe instead of collecting gas over water to prevent solubility loss," or "Use a thermostatically controlled water bath."
Worked Examples of the Hardest IB Chemistry Questions
The following examples represent the toughest types of questions found in the new specimen papers.
Example 1 – HL Calculation Question
This question type tests your ability to manipulate complex thermochemical equations.
The Question
Calculate ΔH for the reaction: CO2 (g) + H2 (g) → CO (g) + H2O (g) given the following data:
Reaction | ΔH / kJ |
2CO (g) + O2 (g) → 2CO2 (g) | -566 |
2H2 (g) + O2 (g) → 2H2O (l) | -572 |
H2O (g) → H2O (l) | -44 |
A. -1182
B. -899
C. -41
D. +41
[1 mark]
Why it’s tricky
The trick is in the state symbols. Equation 2 produces liquid water, but your target equation requires gaseous water. Many students blindly combine equations 1 and 2, completely missing the enthalpy of vaporisation hidden in equation 3.
Worked solution
Step 1: manipulate equation 1:
We need 1 mole of CO2 on the reactants side.
Reverse the equation and adjust the ΔH value:
2CO2 (g) → 2CO (g) + O2 (g) [ΔH = +566 kJ]
Divide the value by 2:
CO2 (g) → CO (g) + ½O2 (g) [ΔH = +566 / 2 = +283 kJ]
Step 2: manipulate equation 2:
We need 1 mole of H2 on the reactants side.
Divide the value by 2:
H2 (g) + ½O2 (g) → H2O (l) [ΔH = −572 / 2 = −286 kJ]
Step 3: Manipulate equation 3:
We need to convert the H2O (l) to H2O (g)
Reverse equation 3 to go from liquid → gas and adjust the ΔH value:
H2O (l) → H2O (g) [ΔH = +44 kJ]
Step 4: Calculate ΔH for the reaction:
+283 − 286 + 44 = +41 kJ mol−1
So, D is the correct answer
Common pitfalls
Cancelling out H2O (l) with H2O (g) as if they are the same species.
Forgetting to flip the sign (+/-) when reversing the equation.
How to succeed
Annotate the question paper.
Circle the state symbols (l) and (g) in the target equation so you don't miss them.
Write out your manipulated equations fully before adding the numbers up.
Example 2 – SL Data-Based Question
This question type tests your ability to deduce chemical properties from raw data (Trends). The difficulty of this question lies in interpreting the drastic jump in ionisation energy data to determine the element's valence state and then predicting the resulting compound formula.
The Question
The table lists successive ionisation energies of an element Z:
Ionisation number | 1st | 2nd | 3rd | 4th | 5th | 6th |
Ionisation energy / kJ mol-1 | 577.54 | 1816.68 | 2744.78 | 11577.5 | 14841.9 | 18379.0 |
Deduce the formula of the stable oxide of element Z.
A. Z2O
B. ZO
C. Z2O3
D. ZO2
[1 mark]
Why it’s tricky
This requires a chain of logic rather than recall. You cannot find the answer in the data booklet. You must analyse the magnitude of the "jumps" between numbers to deduce the electron configuration, then the group number, then the ion charge, and finally the compound formula.
Worked solution
Step 1: Analyse the Data:
Look at the differences between the ionisation energies.
1st to 2nd: ~1300 difference.
2nd to 3rd: ~900 difference.
3rd to 4th: ~8800 difference (Huge jump).
Step 2: Identify the number of valence electrons:
The huge jump occurs after the 3rd electron is removed.
This implies the 4th electron is being taken from a new, inner energy shell closer to the nucleus.
Therefore, Element Z has 3 valence electrons.
Step 3: Deduce the Group & charge of element Z:
It belongs to Group 13 (like Aluminium).
It will form a +3 ion (Z3+)
Step 4: Deduce the formula:
Oxygen forms a -2 ion (O2-)
Two Z3+ ions will balance the charge of three O2- ions
So, the formula is Z2O3
So, C is the correct answer.
Common pitfalls
Counting the jump incorrectly and assuming it is Group 4.
Identifying the Group correctly but writing the formula as ZO3 because you forget oxygen's -2 charge.
How to succeed
Draw a quick sketch of the atom's shells.
Visualise removing the electrons one by one.
The moment you hit the "inner shell," the energy skyrockets.
Count the electrons you removed before that point because that is your Group number.
Example 3 – Practical/Design Question
This question mirrors the new focus on "Inquiry" and experimental validity found in Paper 1B.
The Question
A student is investigating the rate of reaction between magnesium and hydrochloric acid by measuring the volume of hydrogen gas evolved over time using a gas syringe.
Evaluate the validity of the method if the student performs the experiment at 35 oC but uses a gas syringe calibrated at 20 oC.
Suggest one specific improvement to the apparatus to ensure the temperature remains constant.
[3 marks]
Why it’s tricky
This tests "Nature of Science" and error analysis. The command term "Evaluate" requires you to make an appraisal. This means that you cannot just say "it's wrong." You must explain why the discrepancy affects the validity of the specific data being collected (gas volume).
Worked solution
Step 1: Evaluate validity:
The method is invalid because gas volume is temperature-dependent (Charles's Law).
[1 mark]
Hotter gas occupies a larger volume.
If the syringe assumes 20 oC but the gas is 35 oC, you will use the standard molar volume (or the syringe's calibration) to convert this volume into moles
However, accurately measuring a volume expanded by heat will result in a calculated mole value that is artificially high.
This leads to an incorrect calculation of moles of gas produced.
[1 mark]
Step 2: Improvement:
The reaction vessel should be placed in a thermostatically controlled water bath.
This ensures the temperature of the reactants remains constant throughout the experiment, unlike a simple beaker which allows heat loss/gain.
[1 mark]
Common pitfalls
Stating "repeat the experiment" (this improves reliability, not validity).
Ignoring the calibration issue and focusing only on reaction rate.
How to succeed
When asked to evaluate an experiment, check three things:
Control Variables (is temperature/pressure constant?)
Measurement Equipment (is it precise enough?)
Assumptions (is the gas actually ideal?).
Example 4 – HL Organic Mechanism Question
In Organic Chemistry, precision is everything. This question tests your ability to visualise reactions in 3D and applies strictly to Higher Level students.
The Question
Draw the mechanism for the SN2 reaction between bromoethane and sodium hydroxide, specifically showing the transition state.
[3 marks]
Why it’s tricky
This tests the link between Structure (stereochemistry) and Reactivity (mechanisms). The mark scheme is incredibly strict regarding:
The position of curly arrows
The geometry of the transition state
The location of charges.
A correct concept with a sloppy drawing often scores zero.
Worked solution
Step 1: The attack:
Draw a curly arrow originating exactly from the lone pair on the hydroxide ion (:OH−) attacking the carbon atom bonded to the bromine.
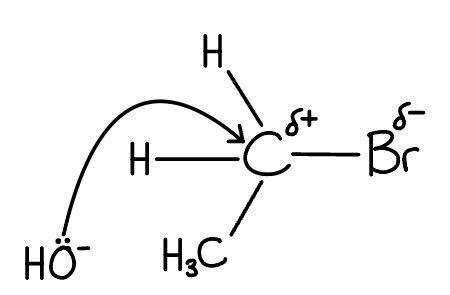
[1 mark]
Step 2: The transition state:
Draw the central carbon atom with dotted lines connecting to both the entering OH group and the leaving Br group.
Draw the other 3 groups (H, H, CH3) attached to the carbon.
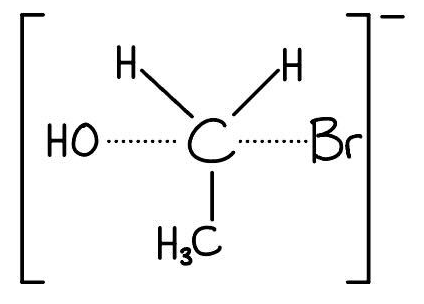
[1 mark]
Crucial:
Enclose the whole structure in large square brackets with a negative charge outside.
Ensure the OH and Br are 180o apart (trigonal bipyramidal arrangement).
Step 3: The leaving group:
Draw a curly arrow from the solid C-Br bond moving onto the bromine atom.
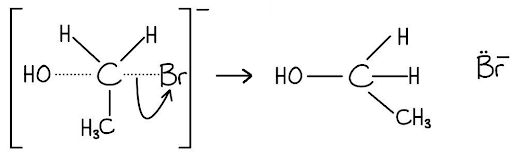
[1 mark]
Common pitfalls
Sloppy arrows: Starting the arrow from the H atom of OH, rather than the lone pair on the Oxygen.
Missing charge: Forgetting the negative sign on the transition state brackets.
Solid bonds: Drawing solid lines instead of dotted lines for the forming/breaking bonds in the transition state.
How to succeed
Practice drawing this specific mechanism until you can do it from memory.
Visualize it as an umbrella turning inside out, i.e. the "Inversion of Configuration."
If your drawing doesn't show this geometric flip, you may lose marks.
How to Improve at High-Level IB Chemistry Questions
To score a 7, you need to move beyond textbook definitions. For a complete guide, check out our article on How to Revise for IB Chemistry.
Master the command terms
The IB is precise. If a question asks you to "Evaluate," you must look at strengths and weaknesses and provide a conclusion. If you only list facts, you are capped at half marks. If it asks you to "Deduce," you must show the logic steps, not just the final answer.
Link topics across the syllabus
The hardest IB Chemistry questions test your ability to see connections.
Equilibrium & Energetics: Activation energy determines reaction rates, while enthalpy and entropy determine equilibrium position.
Organic & Acids: Nucleophiles are Lewis bases; electrophiles are Lewis acids.
Redox & Periodicity: The reactivity series predicts cell potentials.
Learn from Examiner Feedback
Examiner reports are documents written after every exam session detailing exactly where students lost marks. While the raw reports are often restricted to teachers, you can still access this "insider knowledge" through the right resources.
Use Revision Notes with "Examiner Tips"
High-quality revision notes (like those on Save My Exams) are created by analysing these reports.
We extract the recurring "Common Pitfalls" (like forgetting units or sloppy bond drawings) and embed them directly into the notes so you don't make the same mistakes.
Practice with Exam Style Questions
Standard textbook questions often focus on drill-and-practice.
To prepare for the hardest questions, use our Exam Style Questions and Mock Exams.
These are specifically designed to mirror the phrasing, difficulty, and "traps" found in real IB papers.
Test yourself under pressure
Examiner reports consistently state that students underperform because they run out of time.
Use Practice Papers under timed conditions to build the speed and accuracy required for the new calculator-based Paper 1.
Frequently Asked Questions
What are the hardest IB Chemistry topics?
Topics involving abstract maths and 3D visualization are typically the hardest. This includes:
Acids & Bases (Buffers)
Organic Mechanisms
Transition Metals
Energetics (Born-Haber cycles).
Do HL students get different questions than SL students?
Yes. While Paper 1 and 2 share some common questions, HL students face questions that are conceptually deeper.
For example, where SL students calculate pH for strong acids, HL students must calculate pH for weak acid buffers.
How do I improve my evaluation and planning skills?
Focus on the "Why."
When practicing data questions in Paper 1B, don't just calculate the answer; explain why the data looks that way.
Practice identifying systematic vs. random errors in every experiment you review.
How many marks do you need for a 7 in IB Chemistry?
While grade boundaries shift every year, a 7 usually requires approximately 75-80%. With calculators now allowed in Paper 1, boundaries may shift slightly upwards as calculation errors decrease. For more detail, read our guide on how to get a 7 in IB Chemistry.
Final Thoughts
The hardest IB Chemistry questions are designed to distinguish students who truly understand chemistry from those who have merely memorised content. But they follow predictable patterns. By mastering the link between Structure and Reactivity, and practicing the specific technical skills required for mechanisms and graphs, you can turn these "impossible" questions into your greatest opportunity for marks.
Ready to test yourself? Explore our full IB Chemistry Exam Guide for more resources.
Now that you're equipped with the expert strategies to deconstruct and master IB Chemistry's toughest questions, the final step is to put them into practice.
The Save My Exams IB Chemistry library is the perfect place to do this. Our resources are tailor-made to help you apply these new skills and build exam-winning habits:
Solidify your understanding of the core principles with our concise, syllabus-aligned revision notes before you tackle the hard questions.
Topic questions & model answers
Practise the specific types of problems we've covered with our exam-style topic questions.
Each one is created by an expert and comes with a detailed answer, showing you what a top-grade response looks like.
Practice papers and mock exams:
Simulate real exam conditions and perfect your timing with our complete library of practice papers and mock exams written by our specialists in the IB style.
Reinforce your knowledge of definitions, reaction conditions, and colour changes with our interactive flashcards. These are perfect for quick revision sessions on the go.
Stop worrying about the hardest questions and start conquering them today.
References
International Baccalaureate Organisation. (2023). Chemistry guide. Cardiff, Wales: International Baccalaureate Organisation (UK) Ltd.
International Baccalaureate Organisation. (2023). Diploma Programme Assessment procedures.
International Baccalaureate Organisation. (2023). Chemistry specimen papers.
Sign up for articles sent directly to your inbox
Receive news, articles and guides directly from our team of experts.

Share this article
 written revision resources that improve your
written revision resources that improve your