1
1 mark
When 5 g of a fuel was burned, 150 kJ of energy was released.
Calculate the energy, in kJ, that would be released if 60 g of the fuel was burned.
30
150
1800
9000
Was this exam question helpful?
Exam code: X813 75
When 5 g of a fuel was burned, 150 kJ of energy was released.
Calculate the energy, in kJ, that would be released if 60 g of the fuel was burned.
30
150
1800
9000
Choose your answer
Was this exam question helpful?
The apparatus shown was used to measure the energy released when four different fuels were burned.
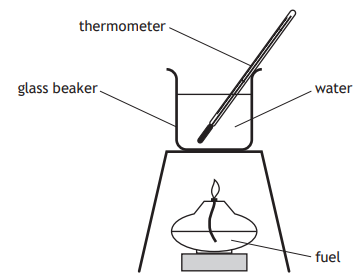
In which experiment was the most energy released?
Mass of water (kg) | Temperature rise (°C) | |
A | 0.05 | 10 |
B | 0.05 | 20 |
C | 0.25 | 8 |
D | 0.25 | 18 |
Choose your answer
Was this exam question helpful?
A student set up an experiment to determine the quantity of energy released when a hydrocarbon burns.
Which of the following diagrams shows the apparatus which would produce the most accurate result?
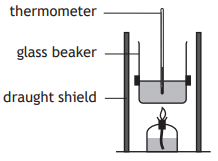
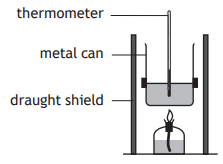
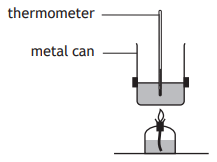
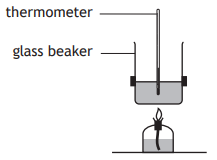
Choose your answer
Was this exam question helpful?