A hammer hits a nail with a force of 5.0 kN.
The pressure exerted by the hammer on the nail is Pa.
The area of the nail hit by the hammer is
Was this exam question helpful?
Exam code: X857 75
A hammer hits a nail with a force of 5.0 kN.
The pressure exerted by the hammer on the nail is Pa.
The area of the nail hit by the hammer is
Choose your answer
Was this exam question helpful?
A sealed hollow buoy drifts from warm Atlantic waters into colder Arctic waters.
The volume of the buoy remains constant.
The pressure of the trapped air inside the buoy changes because pressure is
directly proportional to the temperature in kelvin
inversely proportional to the temperature in kelvin
inversely proportional to the volume of the air inside the buoy
inversely proportional to the temperature in degrees Celsius
directly proportional to the temperature in degrees Celsius
Choose your answer
Was this exam question helpful?
The pressure of a fixed mass of gas is Pa. The temperature of the gas is 320 K and the volume of the gas is 2.2 m3.
The gas is then heated to a temperature of 370 K and the pressure of the gas increases to Pa.
The new volume of the gas is
1.7 m3
2.1 m3
2.3 m3
2.8 m3
4.1 m3
Choose your answer
Was this exam question helpful?
An aircraft is flying at high altitude.
During the flight the pressure of the air inside the aircraft is reduced.
(i) State what is meant by the term pressure.
[1]
(ii) During the flight a passenger notices that the volume of a crisp packet is greater than it was at take‑off.
At take‑off the pressure of the gas inside the crisp packet was 101 kPa and the volume of gas in the crisp packet was 2.3 10−3 m3.
During the flight the pressure of the gas inside the crisp packet is 92 kPa.
The temperature of the gas inside the crisp packet remains constant.
Calculate the volume of the gas inside the crisp packet at a pressure of 92 kPa.
[3]
(iii) Describe how the kinetic model accounts for the pressure of the gas inside the crisp packet.
[1]
How did you do?
Was this exam question helpful?
A cyclist is riding a bicycle along a level road.

The combined mass of the cyclist and bicycle is 70.0 kg.
The total contact area between the tyres and the road is 8.0 10−4 m2.
The average pressure exerted by the tyres on the road is:
1.2 10−6 Pa
5.6 10−2 Pa
8.8 104 Pa
4.3 105 Pa
8.6 105 Pa
Choose your answer
Was this exam question helpful?
The average kinetic energy of a gas molecule can be determined using the following relationship.
where: Ek is the average kinetic energy of a gas molecule in joules, J
kB is Boltzmann’s constant = 1.38 10−23 J K−1
T is the temperature of a gas molecule in kelvin, K.
The average kinetic energy of a gas molecule at 100 °C is:
2.07 10−21 J
3.58 10−21 J
5.15 10−21 J
5.65 10−21 J
7.72 10−21 J
Choose your answer
Was this exam question helpful?
A group of students are investigating how the pressure of a fixed mass of gas varies with its temperature. This is known as Gay‑Lussac’s Law.
The students set up an experiment as shown.
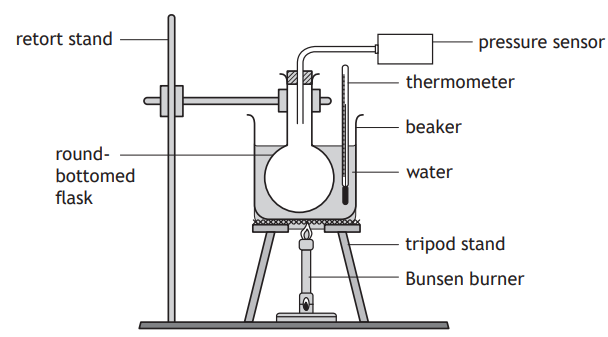
The round‑bottomed flask contains a fixed mass of gas.
The Bunsen burner is used to heat the apparatus as shown. Readings of temperature and pressure are taken every 10 °C.
During the experiment the volume of the gas in the round‑bottomed flask remains constant.
The students’ results are shown.
Temperature (°C) | Temperature (K) | Pressure (kPa) |
50 | 323 | 121 |
60 | 333 | 124 |
70 | 343 | 128 |
80 | 353 | 132 |
Use all the appropriate data to establish the relationship between the pressure and the temperature of the gas.
How did you do?
Predict the pressure of the gas at a temperature of 100 °C.
How did you do?
Suggest one way the students could improve the experiment.
How did you do?
The tyre pressure in racing cars is carefully monitored throughout a race.
As the cars drive around the racing circuit, the temperature of the gas inside the tyres increases.
Explain, using the kinetic model, how this affects the pressure of the gas inside the tyres.
How did you do?
Was this exam question helpful?
The pressure p due to a liquid at a depth h is given by the relationship
where ρ is the density of the liquid and g is the gravitational field strength.
A liquid has a density of 990 kg m-3.
When the pressure due to the liquid is 1470 Pa, the depth in the liquid is
0·069 m
0·15 m
0·67 m
1·5 m
6·6 m
Choose your answer
Was this exam question helpful?
A car is parked in the sun for some time. During this time the air pressure inside the tyres increases.
The reason for this increase in pressure is
the volume occupied by the air particles in the tyres has increased
the force produced by the air particles in the tyres acts over a smaller area
the average spacing between the air particles in the tyres has increased
the increased temperature has made the air particles in the tyres expand
the air particles in the tyres are moving with greater kinetic energy
Choose your answer
Was this exam question helpful?
The temperature of a sample of gas in a container is 20 °C.
The volume of the gas is 0·30 m3.
The container is free to expand in order to maintain a constant pressure.
The temperature of the gas is increased to 50 °C.
The volume now occupied by the gas is
0·12 m3
0·27 m3
0·30 m3
0·33 m3
0·75 m3
Choose your answer
Was this exam question helpful?
A water rocket consists of a plastic bottle partly filled with water. Air is pumped in through the water. When the pressure is great enough, the tube detaches from the bottle. Water is forced out of the bottle, which causes the bottle to be launched upwards.
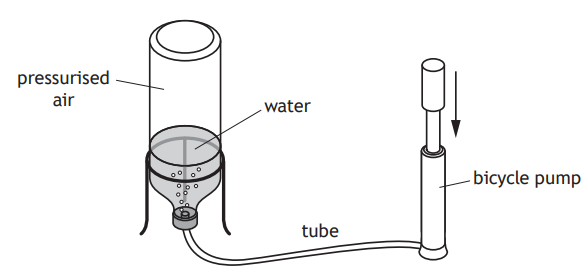
At launch, the air in the bottle is at a pressure of 1·74 105 Pa
The area of water in contact with the pressurised air in the bottle is 4·50 10-3 m2.
Calculate the force exerted on the water by the pressurised air at launch.
How did you do?
At launch, the air in the bottle has a volume of 7·5 10-4 m3.
At one point in the flight, the volume of air in the bottle has increased by 1·2 10-4 m3.
During the flight the temperature of the air in the bottle remains constant.
(i) Calculate the pressure of the air inside the bottle at this point in the flight.
[4]
(ii) Using the kinetic model, explain what happens to the pressure of the air inside the bottle as the volume of the air increases.
[3]
How did you do?
Was this exam question helpful?
A syringe containing air is sealed at one end as shown.

The initial volume of the air in the syringe is 1.40 10−6 m3 and the pressure is 120 kPa.
The plunger is pushed in causing the volume to be reduced by 0.30 10−6 m3.
The temperature of the air inside the syringe remains constant.
The pressure of the air inside the syringe is now
26 kPa
94 kPa
99 kPa
153 kPa
560 kPa
Choose your answer
Was this exam question helpful?
A cyclist inflates the tyres on their bike before going cycling.

The cyclist inflates a tyre to a pressure of 655 kPa.
The temperature of the gas inside the tyre is 21 °C.
At one point in the journey the temperature of the gas inside the tyre is 14 °C.
The mass of gas and the volume of gas inside the tyre remain constant.
(i) Determine the pressure of the gas inside the tyre at a temperature of 14 °C.
[3]
(ii) Explain, using the kinetic model, how the decrease in temperature affects the pressure of the gas inside the tyre.
[3]
How did you do?
At one point, the cyclist stops pedalling and freewheels.

The tyres have a total contact area with the ground of 7.5 10−4 m2 and exert a pressure of 1.02
106 Pa on the ground.
Determine the total mass of the cyclist and bike.
How did you do?
Was this exam question helpful?
The pressure of the air outside an aircraft is 0·40 105 Pa.
The air pressure inside the aircraft cabin is 1·0 105 Pa.
The area of an external cabin door is 2·0 m2.
The outward force on the door due to the pressure difference is
0·30 105 N
0·70 105 N
1·2 105 N
2·0 105 N
2·8 105 N
Choose your answer
Was this exam question helpful?
A solid at a temperature of −20 °C is heated until it becomes a liquid at 70 °C.
The temperature change in kelvin is
50 K
90 K
343 K
363 K
596 K
Choose your answer
Was this exam question helpful?
A sealed bicycle pump contains 4·0 10−5 m3 of air at a pressure of 1·2
105 Pa.
The piston of the pump is pushed in until the volume of air in the pump is reduced to 0·80 10−5 m3.
During this time the temperature of the air in the pump remains constant.
The pressure of the air in the pump is now
2·4 104 Pa
1·2 105 Pa
1·5 105 Pa
4·4 105 Pa
6·0 105 Pa
Choose your answer
Was this exam question helpful?
During the flight the aircraft uses fuel.
Explain why the pressure exerted by the tyres of the aircraft on the runway after the flight is less than the pressure exerted by the tyres on the runway before the flight.
How did you do?
Was this exam question helpful?
A student sets up an experiment to investigate the relationship between the pressure and temperature of a fixed mass of gas as shown.
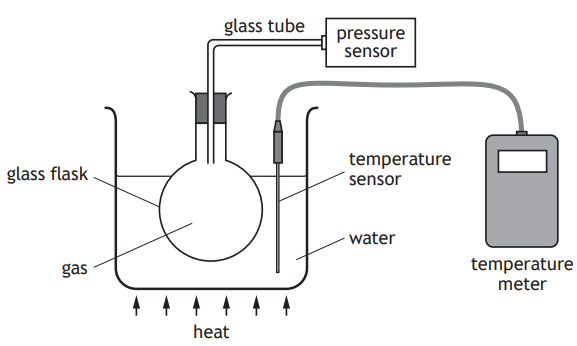
The student heats the water and records the following readings of pressure and temperature.
Pressure (kPa) | 101 | 107 | 116 | 122 |
Temperature (K) | 293 | 313 | 333 | 353 |
(i) Using all the data, establish the relationship between the pressure and the temperature of the gas.
[3]
(ii) Using the kinetic model, explain why the pressure of the gas increases as its temperature increases.
[3]
(iii) Predict the pressure reading which would be obtained if the student was to cool the gas to 253 K.
[1]
How did you do?
State one way in which the set-up of the experiment could be improved to give more reliable results.
Justify your answer.
How did you do?
Was this exam question helpful?