Hardest A Level Chemistry Questions & How to Answer Them
Written by: Richard Boole
Reviewed by: Philippa Platt
Published
Contents
Preparing for A Level Chemistry means facing some genuinely challenging questions. These questions will test the limits of your knowledge and problem-solving ability. The most difficult questions separate A* students from the rest, but is the subject's reputation for being difficult really deserved? For a full statistical breakdown, check out our in-depth guide: Is A Level Chemistry Hard?
As a long-standing GCSE and A-Level Chemistry examiner for both Edexcel and OCR, I've marked thousands of exam scripts and seen exactly where students stumble. Combining that firsthand examiner experience with a deep analysis of official reports, this Save My Exams guide breaks down what makes certain questions so hard and shows you how to tackle them with confidence.
Key takeaways
The hardest A Level Chemistry questions test skills, not just topics. They focus on:
Applying knowledge to unfamiliar situations.
Tackling multi-step calculations.
Analysing data.
Success comes from a systematic approach. Breaking down the question, identifying key information, and showing clear working are essential for securing method marks, even if your final answer is wrong.
This guide uses real exam questions and insights from official examiner reports to show you exactly what top-grade answers look like and the common pitfalls to avoid.
Why are some A Level Chemistry questions so difficult?
To understand what makes a question hard, you need to know what examiners are testing. Your final grade is determined by your performance across three Assessment Objectives (AOs). Here's what they mean in practice:
AO1: Knowledge and understanding
This tests what you can remember, such as:
Definitions, e.g. 'State what is meant by...'
Equations
Reaction conditions.
These are typically the more straightforward marks.
AO2: Application
This tests if you can use your knowledge in new situations. This could involve:
Solving a calculation
Applying a principle to an unfamiliar context
Interpreting data
AO3: Analysis and evaluation
This tests your ability to:
Dissect information
Evaluate experimental methods
Identify patterns
Draw conclusions
The hardest questions are deliberately designed to be heavy on AO2 and AO3. They test whether you can think like a chemist, not just remember facts.
So, what do these AO2 and AO3 questions look like in a real exam?
We've analysed years of official examiner reports, and the toughest questions consistently fall into one of these five categories:
1. Application to unfamiliar contexts
This involves taking a known concept and applying it to a novel scenario that isn't explicitly in the textbook. Examples include:
Mechanisms
Equilibrium principles
2. Multi-step, unstructured calculations
These are problems that require you to combine multiple calculation steps where the path is not immediately obvious. Examples include:
Back titrations
Multi-stage yield calculations
Combining gas laws with stoichiometry
3. Synoptic "Levels of Response" questions
These questions require you to pull together information from multiple different areas of the specification into a coherent, written answer. Examples include:
Practical techniques
Structure and bonding
Energetics
4. Data interpretation & practical skills
This includes:
Reading graphs
Interpreting spectra
Understanding the why behind practical steps (not just the what)
Dealing with percentage uncertainty
5. Precision in language
These are questions that require deep, precise explanations. This means that the common, vague student answers are not enough to score the top marks. Examples include:
Explaining electrode potentials
Buffer action
Trends in bonding
Before we dive into the examples, it's crucial to know your exam board. The structure of the papers differs between AQA, OCR, and Edexcel. This will affect where you're most likely to encounter these challenging questions. For a full breakdown, check out our guide on: How many A Level Chemistry papers there are?
Examples of the hardest A Level Chemistry questions
These examples illustrate the five toughest types of questions you'll encounter and show you how to build your answer.
Example 1: Applying rules to unfamiliar contexts
This first type of question tests your ability to apply fundamental principles to a reaction you've never seen before.
Here’s a classic mechanism problem from an OCR paper.
The question gives information about the reaction of a benzenediazonium ion, including the following reaction steps:
Step 1: Elimination of nitrogen gas to form a carbocation
Step 2: Nucleophilic attack by water
Step 3: Proton loss to form the organic product
It then asks you to:
“Complete the boxes below with intermediates and curly arrows to show the mechanism for this reaction.”

[4 marks]
(Source: OCR A-Level Chemistry A - H432/03 - Summer 2023, Q2c (opens in a new tab))
Why it's tricky:
Examiners love to present reactions you've never seen before.
The 2023 OCR Examiner Report notes that many students panicked and relied on the familiar electrophilic substitution mechanism. It suggests that this mechanism was memorised for questions involving aromatic compounds. However, the electrophilic substitution mechanism was not relevant and scored no marks.
What the examiner is looking for:
The examiner was looking for students to:
Apply the fundamental rules of curly arrows and reaction types (like nucleophilic attack)
Follow a set of instructions
They did not want a rote answer, recalling a specific mechanism from a textbook.
Worked solution:
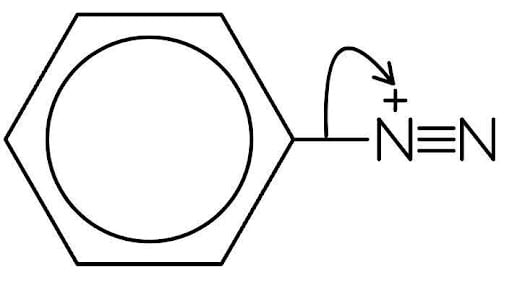
Step 1: Elimination of nitrogen gas to form a carbocation
The C-N bond breaks, with a curly arrow starting from the bond and pointing to the nitrogen atom.
This eliminates a stable N2 molecule and forms a highly reactive phenyl carbocation.
Step 2: Nucleophilic attack by water
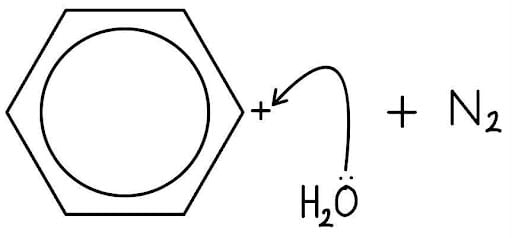
A water molecule acts as a nucleophile.
The curly arrow starts from a lone pair on the oxygen atom.
The curly arrow points to the positively charged carbon of the ring, forming a new C-O bond.
This creates a positively charged oxonium ion intermediate.
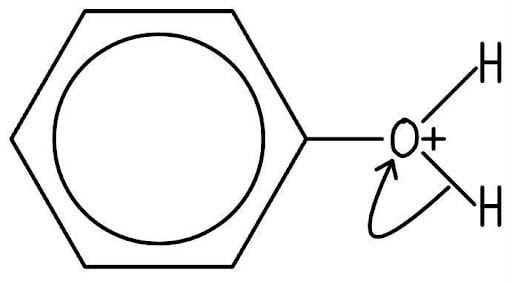
Step 3: Proton loss to form the organic product
To form the neutral phenol product, a proton (H+) is lost.
The curly arrow starts from the O-H bond and points to the positively charged oxygen atom, neutralising it.
Common pitfalls:
Using the wrong mechanism
Don’t try to force a memorised mechanism where it doesn't belong.
Incorrect curly arrows
Make sure curly arrows are correct.
Remember the golden rule:
A curly arrow shows the movement of an electron pair and must start from a source of electrons (a lone pair or a bond).
For this question, a common error was drawing the arrow for proton loss starting from the wrong place.
Forgetting charges
Make sure all full and partial charges are included.
For this question, this referred to the positive charges on the carbocation and oxonium ion intermediates.
How to succeed:
Trust the question
The prompt often tells you exactly what to do.
Your job is to translate the words into the precise language of structures and arrows.
Apply the principles, don't just memorise
Understand what a nucleophile is and how curly arrows work.
This allows you to tackle any reaction thrown at you.
Example 2: Tackling multi-step calculations (The unit trap!)
A-Level Chemistry is full of multi-step calculations. Examiners know that the hardest questions aren't always the ones with complex chemical principles. They are the ones that test your attention to detail under pressure. They will often include "hidden traps". These test whether you are just following a formula or if you truly understand the concepts and units you are working with.
Here's a great example from an Edexcel paper:
Calculate the enthalpy change for the reaction shown.
H2 (g) + ½O2 (g) → H2O (g)
Data:
H2 (g) + ½O2 (g) → H2O (l) ΔH1θ = –285.8 kJ mol–1
H2O (l) → H2O (g) ΔH2θ = +2.261 kJ g–1
[3 marks]
(Source: Edexcel A-Level Chemistry (8CH0) - Summer 2023, Paper 02, Q8bii (opens in a new tab))
Why it's tricky:
Your teachers are right that units are important!
The 2023 Edexcel Examiner Report highlighted that this question contained a deliberately mismatched unit
The first enthalpy value was given in kJ mol-1
The second enthalpy value was given in kJ g-1
The report noted: "the stronger candidates identified this problem and made an appropriate correction." Many students who tried to combine the numbers directly without converting the units lost marks.
What the examiner is looking for:
The examiner was looking for students to:
Check for consistency in units before starting a calculation
Carry out a logical, multi-step Hess's Law problem
Worked solution:
Step 1: Spot the trap & convert the units
Before starting, all data must be in kJ mol-1:
molar mass of H2O = (2 x 1.0) + 16.0 = 18.0 g mol-1
enthalpy of vaporisation = +2.261 kJ g-1 x 18.0 g mol-1
enthalpy of vaporisation = +40.698 kJ mol-1
Step 2: Construct the Hess's Law Cycle
The target reaction is the formation of gaseous water. The data provides a two-step indirect route via the formation of liquid water first.
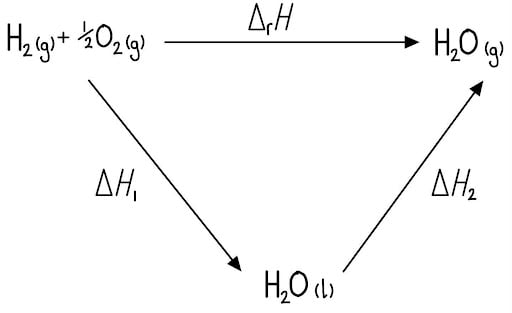
Step 3: Apply Hess's Law
According to Hess's Law, the direct route is equal to the sum of the steps in the indirect route. So, the equation is:
ΔfH (direct route) = ΔH1 + ΔH2 (indirect route)
ΔfH (direct route) = (–285.8) + (+40.698)
ΔfH (direct route) = –245.102 kJ mol-1
Rounding to four significant figures (consistent with the data provided):
ΔfH (direct route) = –245.1 kJ mol-1
Common pitfalls:
Missing the unit trap
Using +2.261 in the final calculation without converting it to a molar value.
This was the biggest single error and meant that students could not get a correct final answer!
Incorrect application of Hess's Law
Getting the direction of the cycle wrong or subtracting when you should add.
Drawing the cycle clearly helps to prevent this.
Arithmetic errors
Simple calculator mistakes under pressure are common.
Always double-check your calculation.
How to succeed:
Always check the units
Before starting any calculation, scan all the data and check that everything is in consistent units (e.g., kJ mol⁻¹, grams, cm³).
This is the number one rule for avoiding hidden traps.
Draw the cycle clearly
Don't do it in your head.
A clear diagram with arrows showing the correct direction will:
Guide your calculation.
Make the relationship between the enthalpy values obvious.
Example 3: Tackling synoptic written questions (The comparison challenge!)
Some of the most daunting questions in an A-Level paper are the 6-mark "Level of Response" (LoR) / “Extended Response” questions. These questions aren't just about recalling facts. They test your ability to:
Build logical, detailed, and well-structured arguments.
Connect ideas from different parts of the specification.
This example Edexcel question asks you to:
“Explain the difference in the reactivity of bromine with benzene and with phenol. Include the type of reaction, the products that form, and any conditions required."
[6 marks]
(Source: Edexcel A-Level Chemistry (9CH0) - Summer 2022, Paper 03, Q5 (opens in a new tab))
Why it's tricky:
This 6-mark question is a classic synoptic comparison that requires a structured, comparative argument.
The 2022 Edexcel Examiner Report states that many students can state basic facts about each substance (e.g., "phenol is more reactive"). But, few students can:
Effectively link ideas together to explain the difference in reactivity, conditions, and products.
Construct a complete, comparative argument that links the cause to the consequences.
What the examiner is looking for:
A well-structured answer that moves logically from the cause of the reactivity difference to the resulting consequences for the reaction conditions and the final products. They are testing your ability to compare and contrast, not just list facts.
Worked solution:
For a 6-mark written question, the "solution" is a well-structured plan. Here’s how to build a top-band answer to this question:
Step 1: Explain the reactivity difference (The 'why')
Start by stating that phenol is more reactive than benzene towards electrophiles like bromine.
The reason for this is that the lone pair of electrons in the p-orbital of phenol's oxygen atom is delocalised into the pi-system of the ring.
This delocalisation increases the electron density of the ring.
This makes it much more attractive to attacking electrophiles.
The -OH group is described as an "activating group".
Step 2: Compare the reaction conditions and type
Explain that because of its lower electron density, benzene requires:
A halogen carrier catalyst (e.g., FeBr3).
Heat to polarise the Br2 molecule and create a strong enough electrophile.
In contrast:
Phenol's ring is so strongly activated that it can polarise the Br2 molecule without a catalyst.
The reaction occurs readily with aqueous bromine (bromine water) at room temperature.
State that for both molecules, the reaction type is electrophilic substitution.
Step 3: Compare the products
The reaction with benzene:
Results in mono-substitution.
Forms a single product: bromobenzene (and HBr).
The reaction with phenol:
Is so vigorous that tri-substitution occurs.
Forms a white precipitate of 2,4,6-tribromophenol, along with three molecules of HBr.
Common pitfalls:
Lack of comparison
Discussing benzene and phenol in two separate paragraphs without explicitly drawing links between them.
For example, "Benzene needs a catalyst, whereas phenol does not..."
Vague explanations
Examples include:
Stating "phenol is more reactive" without explaining the role of the oxygen's lone pair and increased electron density.
Saying "the -OH group gives electrons to the ring" without mentioning the lone pair on the oxygen atom and its delocalisation.
Incorrect products / conditions
Examples include:
Forgetting the halogen carrier for benzene.
Stating that phenol only forms one product.
Forgetting that 2,4,6-tribromophenol is an observable white precipitate.
How to succeed:
Structure your comparison
Plan your answer around three key points:
Reactivity
Conditions
Products
Address each point for both benzene and phenol to ensure your answer is comparative.
Use keywords
Make sure your explanation includes key scientific terms like:
Lone pair
Delocalisation
Pi-system
Electron density
Halogen carrier
Example 4: Interpreting data (The hidden information!)
Some of the toughest calculation questions aren't about using obscure formulae. They're about carefully interpreting a practical scenario and tracking every substance involved, including the ones that don't react.
This AQA gas stoichiometry question is a classic example:
A sample of butane has a volume of 20 cm3 at room temperature and pressure. The sample is burned completely in 1350 cm3 of air. The final mixture is cooled to room temperature and pressure.
C4H10 + 6½O2 ⟶ 4CO2 + 5H2O
Calculate the total volume of gas in the final mixture.
Assume that air contains 21% by volume of oxygen.
[4 marks]
(Source: AQA A-Level Chemistry (7405) - June 2024, Paper 2, Q03.4 (opens in a new tab))
Why it's tricky:
This question is a minefield of potential errors because you have to account for everything. It's not just about what's produced; it's about what's left over.
The 2024 AQA Examiner Report notes that students lost marks by:
Forgetting about the unreacted, inert components of a mixture (like nitrogen in air).
Failing to identify the limiting reactant correctly.
Not considering the final state of the products (e.g., is water a gas or a liquid?).
What the examiner is looking for:
A clear, logical method that accounts for all gaseous species at the start and at the end. They are testing:
Your ability to break a complex problem down into manageable steps.
Your attention to detail.
Worked solution:
This is a limiting reactant problem involving gases. The key is to track the volume of each gas before, during, and after the reaction.
Step 1: Calculate the initial volumes of all gases
Butane (C4H10) = 20 cm3
Oxygen (O2): The air is 21% oxygen.
So, the volume of O2 is:
0.21 x 1350 cm3 = 283.5 cm3
Nitrogen (N2): Air is roughly 79% nitrogen.
N2 is inert and will not react!
So, the volume of N2 is:
0.79 x 1350 cm3 = 1066.5 cm3
Step 2: Identify the limiting reactant
The ratio of C4H10 : O2 is 1 : 6.5.
So, the volume of O2 required for combustion is:
20 cm3 of C4H10 x 6.5 = 130 cm3
There is 283.5 cm3 of O2, but the reaction only requires 130 cm3.
Therefore, oxygen is in excess and butane is the limiting reactant.
All calculations must be based on butane.
Step 3: Calculate the volume of gaseous products and leftover reactants
Volume of CO2 produced:
The ratio of C4H10 : CO2 is 1 : 4.
So, the volume of CO2 produced is:
20 cm3 of C4H10 x 4 = 80 cm3
Volume of H2O produced:
The question states the final mixture is cooled to room temperature.
At this temperature, water is a liquid.
So, its volume as a gas is negligible and can be considered 0 cm3.
This is a crucial detail.
Volume of unreacted O2:
volume left = initial volume - volume used
unreacted O2 = 283.5 cm3 - 130 cm3 = 153.5 cm3
Volume of unreacted N2:
Nitrogen is inert.
So, its volume doesn't change.
unreacted N2 = 1066.5 cm3
Step 4: Calculate the total volume of gas in the final mixture
total volume = volume(CO2) + volume(unreacted O2) + volume(unreacted N2)
total volume = 80 + 153.5 + 1066.5 = 1300 cm3
Common pitfalls:
Forgetting nitrogen
The single most common error is forgetting that the unreactive nitrogen from the air is still present in the final mixture.
Including water's volume
Including the volume of water as a gas.
The mixture was cooled to room temperature where H2O is a liquid, which means that it has no volume.
Stoichiometry errors
Using the wrong volume ratio from the balanced equation.
Limiting reactant mistakes
Assuming all the oxygen reacts.
It is essential to calculate which reactant runs out first.
How to succeed:
Use a table
For complex gas calculations, a simple table can help you keep track.
Create rows for each substance (C4H10, O2, N2, CO2, H2O)
Create columns for "Initial Volume," "Change," and "Final Volume."
Read the conditions
Pay extremely close attention to the conditions, especially the final temperature and pressure.
This often tells you the state of the products.
Example 5: Using precise language (The A* detail!)
At the highest level, A-Level Chemistry isn't just about getting the right answer; it's about explaining why it's the right answer with scientific precision. Questions that ask you to "suggest reasons" or "explain" are designed to test the depth of your conceptual understanding.
For example, this AQA question asks for “reasons for the differences between the values obtained”
Table 3 shows values, obtained by different methods, for the enthalpy of combustion of a different liquid hydrocarbon.
Table 3
Method | Enthalpy of combustion / kJ mol–1 |
1. Standard enthalpy of combustion ΔcHθ298 | –4194 |
2. Value calculated from a calorimetry experiment | –1100 |
3. Value calculated using mean bond enthalpies | –3159 |
Suggest reasons for the differences between the values obtained by each of Methods 2 and 3, and the value obtained by Method 1 in Table 3.
[5 marks]
(Source: AQA A-Level Chemistry (7405) - June 2024, Paper 2, Q11.2 (opens in a new tab))
Why it's tricky:
This question demands a deep and precise understanding of thermodynamic definitions.
The June 2022 AQA Examiner Report noted that:
Many students could give simple answers
But, "very few recognised that a calculation based on mean bond enthalpies does not account for the enthalpy changes in the transition from a liquid to a gaseous state."
What the examiner is looking for:
A detailed explanation that goes beyond the obvious. They want to see that you:
Demonstrate a full understanding of the conditions and states that apply to different thermodynamic quantities.
Understand the limitations of both experimental methods and theoretical models.
Can articulate these limitations using precise scientific language.
Worked solution:
A top-grade answer will address both comparisons (Method 2 vs 1 and Method 3 vs 1) separately and with precision.
Step 1: Explaining the calorimetry result (Method 2 vs. Method 1)
Start by stating the observation:
The experimental value (–1100 kJ mol-1) is significantly less exothermic than the standard data book value (–4194 kJ mol-1).
Explain the main reasons for this discrepancy, which are systematic experimental errors:
Heat loss to the surroundings:
A significant amount of the heat released by the combustion is lost to the air and the apparatus, instead of heating the water.
This is the largest source of error.
Incomplete combustion:
The fuel may not burn completely, producing carbon monoxide (CO) or soot (C) instead of just CO2.
This releases less energy per mole of fuel than complete combustion.
Non-standard conditions:
The experiment is not carried out under perfectly standard conditions.
Step 2: Explaining the bond enthalpy result (Method 3 vs. Method 1)
State the two key theoretical reasons for the difference:
Mean bond enthalpies are averages:
Mean bond enthalpies are averaged values taken from a wide range of different molecules.
They do not represent the exact bond enthalpy of the C-H, C-C, and O-H bonds within the specific molecules of this reaction.
Difference in physical states (The A* Point):
This is the most crucial point.
Bond enthalpy is defined as the energy to break one mole of bonds in the gaseous state.
However, standard enthalpy of combustion (ΔcHθ) involves substances in their standard states.
In the combustion of a liquid hydrocarbon, the fuel (liquid) and the water produced (liquid) are not gases.
The bond enthalpy calculation does not account for the energy changes of vaporisation, leading to an inaccurate result.
Common pitfalls:
Generic answers
Only stating that "mean bond enthalpies are averages" without mentioning the crucial point about state symbols.
Confusing the errors
Applying experimental errors like "heat loss" to the theoretical bond enthalpy calculation.
Vague language
Using imprecise terms like "the numbers are different" instead of specific ones like "less exothermic."
How to succeed
Always think about definitions
When you see a term like "Standard Enthalpy of Combustion" or "Mean Bond Enthalpy," ask yourself: what are the precise definitions, including the required conditions and state symbols?
This will often reveal the source of any discrepancies.
Be specific
For "suggest reasons" questions with multiple marks, provide distinct, well-explained points.
For this question, the two separate experimental errors are:
Heat loss.
Incomplete combustion.
The two separate theoretical reasons are:
Averages.
State differences.
Your strategy guide for exam success
Developing a systematic approach to challenging questions dramatically improves your success rate and reduces exam anxiety.
Break down the question
Start by reading the entire question carefully.
Identify what you're actually being asked to find or explain.
Circle or underline key information, data, and command words like "Explain," "Calculate," or "Evaluate."
Always show your working
For calculations:
Write down equations before substituting numbers.
Show all your steps.
Clear working allows examiners to award "error carried forward" marks, so you can still get credit even if you make an early mistake.
"The biggest mistake students make is not showing their working. For a multi-step calculation, even if the final answer is wrong, we can award method marks for the steps you got right. A clear, logical method could be the difference between a C and an A grade."
Use precise scientific language
Vague language loses marks.
Use the precise chemical terms you've learned.
For example, explain why phenol is more reactive by referencing the "lone pair on the oxygen atom delocalising into the pi-system." Do not just say it "gives electrons."
Watch the clock
Use the number of marks as a guide for your timing (approx. 1 minute per mark).
If you're stuck on a 2-mark question for five minutes:
Make your best attempt.
Flag it.
Move on.
You can always come back at the end if you have time.
How to practise for the hardest questions
Strategic practice is the secret to turning hard questions into easy marks.
Focus on past papers and examiner reports
Work through challenging questions from your specific exam board under timed conditions. This builds both skill and stamina.
Analyse mark schemes
Don't just check if your answer was right or wrong. The mark scheme reveals the specific phrases and concepts that examiners are looking for.
Examiner reports are even better, as they explain the common mistakes students make so you can avoid them.
Target your weak topics
Go through the specification and RAG rate each topic:
Red (weak)
Amber (okay)
Green (confident).
Focus your time on turning your red topics to amber. You can find a complete checklist for your exam board in our full guide to A-Level Chemistry Topics by Exam Board.
Build a "tricky questions" list
This is a game-changing revision technique. Every time you get a question wrong in practice:
Write down the question.
Briefly explain why you got it wrong.
Write out the correct approach.
Revisit this list every week. You will quickly turn your weaknesses into strengths.
Putting these strategies into action requires a plan. For a complete guide on how to schedule your revision effectively and use memory techniques, read our step-by-step article on: How to make an A-Level revision timetable.
Frequently asked questions
What are the hardest topics in A Level Chemistry?
While this can vary from student to student, our analysis shows that the "hardest" parts of chemistry aren't always specific topics, but types of questions. The most challenging questions, which consistently appear across all exam boards, are those that require:
Synoptic questions that combine multiple topics (like equilibrium and kinetics).
Multi-step calculations.
Complex spectroscopy analysis.
How do I know if a question is high-level?
High-level questions are less about simple recall (AO1) and more about application and analysis (AO2 & AO3). You can spot them by looking for:
Key command words: "Evaluate," "Compare," "Explain your reasoning," or "Deduce using the data..."
Unfamiliar contexts or scenarios you haven't seen before.
Multiple steps or a high mark allocation (e.g., 4-6 marks).
How can I boost my confidence with hard questions?
Confidence is built through smart, consistent practice. Try these proven methods:
Start with manageable questions to build momentum before tackling the hardest ones.
Use active recall: Test yourself without looking at your notes. This is scientifically proven to build stronger memories.
Teach the concept to someone else. Explaining a difficult topic aloud is the ultimate test of your own understanding and quickly reveals any gaps.
Do all papers have really hard questions?
Yes, all A Level Chemistry papers are designed with a range of difficulty to discriminate between grades. The hardest questions are there to identify the A/A* candidates. They typically appear towards the end of the paper.
Final thoughts
The hardest A Level Chemistry questions are designed to make you think, not just remember. They require genuine understanding, creative application of principles, and sharp analytical skills.
Approaching these questions strategically makes them far more manageable:
Break them down.
Apply systematic methods.
Practice with real past papers and examiner reports.
Don't view difficult questions as a barrier. See them as an opportunity to demonstrate your mastery of the subject. With the techniques in this guide, you'll be ready to tackle them with confidence.
Mastering these questions is a key part of securing a top grade. For a complete roadmap covering revision planning and effective study techniques, read our comprehensive guide on: How to Get an A in A-Level Chemistry.
Now that you're equipped with the expert strategies to deconstruct and master A-Level Chemistry's toughest questions, the final step is to put them into practice.
The Save My Exams A-Level Chemistry library is the perfect place to do this. Our resources are tailor-made to help you apply these new skills and build exam-winning habits:
Revision notes
Solidify your understanding of the core principles with our concise, syllabus-aligned revision notes before you tackle the hard questions.
Topic questions & model answers
Practise the specific types of problems we've covered with our exam-style topic questions.
Each one is created by an expert and comes with a detailed answer, showing you what a top-grade response looks like.
Past papers:
Simulate real exam conditions and perfect your timing with our complete library of past papers and mark schemes from the AQA, Edexcel and OCR exam boards.
Stop worrying about the hardest questions and start conquering them today.
References:
AQA (Assessment and Qualifications Alliance)
AQA A-Level Chemistry (7405), Paper 2: Organic and Physical Chemistry, June 2024 series (opens in a new tab)
AQA A-Level Chemistry (7405), Examiner Report, Paper 2: Organic and Physical Chemistry, June 2024 series (opens in a new tab)
Edexcel (Pearson)
Pearson Edexcel AS-Level Chemistry (8CH0), Paper 02: Core Organic and Physical Chemistry, Summer 2023 series (opens in a new tab)
Pearson Edexcel AS-Level Chemistry (8CH0), Principal Examiner Feedback, Paper 02: Core Organic and Physical Chemistry, Summer 2023 series (opens in a new tab)
Pearson Edexcel A-Level Chemistry (9CH0), Paper 03: General and Practical Principles in Chemistry, Summer 2022 series (opens in a new tab)
Pearson Edexcel A-Level Chemistry (9CH0), Examiners' Report, Paper 03: General and Practical Principles in Chemistry, Summer 2022 series (opens in a new tab)
OCR (Oxford Cambridge and RSA Examinations)
Was this article helpful?
Sign up for articles sent directly to your inbox
Receive news, articles and guides directly from our team of experts.

Share this article
 written revision resources that improve your
written revision resources that improve your