Hardest GCSE Chemistry Questions & How to Answer Them
Written by: Philippa Platt
Reviewed by: Richard Boole
Published
Contents
- 1. Key Takeaways
- 2. Why Some GCSE Chemistry Questions Are So Hard
- 3. How to Approach Difficult Chemistry Questions
- 4. Hardest GCSE Chemistry Questions by Topic
- 5. Extended response questions
- 6. Exam Tips
- 7. Common Mistakes Students Make (and How to Avoid Them)
- 8. Frequently Asked Questions
- 9. Final Thoughts
- 10. References:
Struggling when those really tough Chemistry questions appear in your GCSE exam? Trust me, you’re not the only one! Every year, students across the UK come up against questions that make them hesitate, doubt themselves, or wonder whether they’ve even revised the right topic.
But is the subject's reputation for being difficult really deserved? For a teacher's perspective on this, check out our in-depth guide: Is GCSE Chemistry Hard?.
As a chemistry teacher with nearly 20 years of classroom experience, I’ve seen exactly where students slip up, and more importantly, what helps them overcome those tricky moments. The good news? With the right approach and a bit of guided practice, even the hardest GCSE Chemistry questions become completely manageable.
Let's look at why some questions feel so challenging and how you can answer them with confidence.
Key Takeaways
Before we get into the hardest question types, there are a few golden rules I always share with my classes:
Command words are your secret weapon
Words like “explain”, “evaluate” and “justify” are clues.
They tell you exactly how much detail to give and what kind of answer the examiner is looking for.
Always show your working
Don’t just write the final number.
If the method is there, you can still pick up marks even if the final answer isn’t perfect.
Good structure = easier marks
Set out your answer in clear, logical steps or short paragraphs.
This makes it much easier for the examiner to follow your thinking and reward you for it.
Weird-looking questions aren’t a trap
When a question is set in an unfamiliar context, it’s not there to trick you.
It’s just checking whether you can use your chemistry knowledge in a new situation.
The science underneath is still the same.
Why Some GCSE Chemistry Questions Are So Hard
Let’s be real, not all Chemistry questions are made equal. Some you can answer without thinking, while others feel like they’ve been written in code. So what actually makes certain GCSE Chemistry questions so challenging?
They demand multi-step thinking
The toughest questions rarely test just one skill. Instead, they expect you to link several ideas together.
You might need to recall a definition, carry out a calculation, and explain your reasoning, all in the same answer.
They rely on abstract concepts
Topics like equilibrium or atomic structure involve particles and processes you can’t see. Unlike a simple classroom experiment, these ideas are theoretical, which can make them harder to picture and understand.
They use unfamiliar contexts
Examiners love placing familiar chemistry into unfamiliar scenarios, unusual reactions, industrial settings, or real-world applications. This isn’t meant to throw you off; it’s a way of checking whether you can apply your understanding, not just memorise facts.
The mark scheme is detailed
Higher-mark questions often have several specific marking points. Even if you understand the idea, missing a key term or explanation can mean missing out on marks.
Calculations become more complex
It’s not just about choosing the right formula.
You may need to rearrange equations, convert units, use significant figures correctly, and keep your working clear, all while under time pressure.
The upside?
Once you understand why these questions are difficult, you can use targeted strategies to overcome them. And that’s where real progress begins.
How to Approach Difficult Chemistry Questions
Okay, time to get practical. When you’re staring at a question that makes your brain ache, use this step-by-step game plan:
1. Read the question twice (properly!)
Don’t skim. The fastest way to lose marks is to miss a tiny detail. On your second read, underline or highlight key bits like numbers, substances, and any specific instructions.
2. Crack the command word
What is the question actually asking you to do, describe, explain, or evaluate? Each one needs a different style of answer:
Describe = Say what you see or what happens.
Explain = Say why it happens (using words like “because” or “so”).
Evaluate = Weigh up pros and cons or make a judgement about something.
3. Look at the marks available
Circle the number of marks. A 1-mark question needs one clear point. A 6-mark question needs several points or a detailed, layered answer. Let the marks guide how much you write.
4. Figure out what you’re dealing with
What information have they given you? Is there data, a table, a graph? Which formula might you need? Which topic is being tested? Often, just identifying the topic (e.g. electrolysis, moles, bonding) unlocks the method.
5. Plan your answer in your head first
Don’t rush straight into writing. Spend 10–15 seconds deciding what you’re going to do.
For calculations: jot down the formula first.
For longer answers: quickly think through the key points you need to include.
6. Show every step of your working
Even if you’re sure you’re right, write out each stage clearly. If you slip up on one small step, you can still earn method marks for the rest of your working.
7. Ask: “Does this answer make sense?”
Check your final result.
A negative temperature?
A pH of 25?
A mass larger than what you started with?
Those are red flags. If something looks unrealistic, go back and hunt for a mistake.
Before we dive into the examples, it's crucial to know your exam board. The structure of the papers differs between AQA, OCR, and Edexcel. This will affect where you're most likely to encounter these challenging questions. For a full breakdown, check out our guide on: How many GCSE Chemistry papers are there?
Hardest GCSE Chemistry Questions by Topic
Let's tackle some genuinely challenging questions across the key topics. These are based on real past paper questions that students typically find tough.
Titration calculations
Calculation questions can feel overwhelming because they often combine several steps. This example using a titration shows how to tackle them one stage at a time, so the maths becomes much more manageable.
A student does a titration using sodium carbonate solution and nitric acid.
The equation for the reaction is:
Na2CO3 + 2HNO3 → 2NaNO3 + CO2 + H2O
25.0 cm3 of 0.124 mol / dm3 sodium carbonate solution is neutralised by 23.6 cm3 of nitric acid.
Calculate the concentration of the nitric acid. Give your answer to 3 significant figures.
[5 marks]
(Source: AQA GCSE Chemistry Paper (Higher) 1 June 2023, Q5.5 (opens in a new tab))
Why is this hard?
This question involves three linked calculation steps, each of which must be correct to reach the final answer. Many students lose marks by:
Forgetting to convert cm3 to dm3
Not applying the 1:2 mole ratio correctly
Dividing by volume in the wrong units
Not showing enough working for method marks
It’s a typical “chain calculation” where one small slip can affect the final concentration.
How to answer it:
Make sure all the units are the ones you need to use, e.g. cm3 not dm3
Calculate the moles of Na2CO3
Use the ratio
Calculate the concentration of HNO3
Model answer:
Step 1: Convert the volume of Na2CO3 to dm3:
25.0 cm3 ÷ 1000 = 0.0250 dm3
Step 2: Calculate moles of Na2CO3:
moles = concentration x volume
moles of Na2CO3 = 0.124 mol / dm3 x 0.0250 dm3
moles of Na2CO3 = 0.00310 mol
Step 3: Use the mole ratio:
The equation shows Na2CO3 : HNO3 = 1 : 2.
So, the moles of HNO3 needed is:
2 x 0.00310 mol = 0.00620 mol
Step 4: Convert the nitric acid volume to dm3:
23.6 cm3 ÷ 1000 = 0.0236 dm3
Step 5: Calculate the concentration of HNO3:
concentration = moles / volume
concentration of HNO3 = 0.00620 ÷ 0.0236
concentration of HNO3 = 0.263 mol / dm3
Top tip:
Always convert cm3 → dm3 before calculating moles.
Nearly all lost marks in titration questions come from mixing units.
Write “÷1000” on the page as a reminder before you start.
Bonding and structure
Structure and bonding questions often require precise scientific language. This example helps you practise describing a substance clearly using the correct key terms.
Example question
This question is about different forms of carbon.
Figure 5 represents the structure of diamond.
Figure 5

Explain why diamond has a very high melting point.
[3 marks]
(Source: AQA GCSE Chemistry Paper (Higher) Paper 1 June 2022, Q3.2)
Why this is hard:
Students often mix up covalent bonds with intermolecular forces. Diamond is a giant covalent structure, not a simple molecule, so there are no intermolecular forces at all. Misusing terms (e.g., “more energy is needed” with no comparison) costs marks.
How to answer it:
State that diamond is a giant covalent lattice.
Each carbon makes four strong covalent bonds.
Explain that these strong bonds must be broken to melt diamond, requiring a lot of energy.
Model answer:
Diamond is a giant covalent lattice where each carbon atom forms four strong covalent bonds.
To melt diamond, a very large amount of energy is needed to break these many strong bonds.
Top tip:
If the whole substance is a single three-dimensional giant covalent network (like diamond, silicon dioxide), never mention intermolecular forces.
If the substance has giant covalent layers (like graphite), you must mention the weak intermolecular forces between the layers.
Electrolysis
Electrolysis questions can seem confusing because several ions are involved at once. This example focuses on breaking the process down so you can describe what happens at each electrode.
This question is about electrolysis. A student investigated the electrolysis of copper chromate solution.
Copper chromate solution is green.
Copper chromate contains:
blue coloured Cu2+ ions
yellow coloured CrO42- ions.
Figure 1 shows the apparatus used.
Figure 1
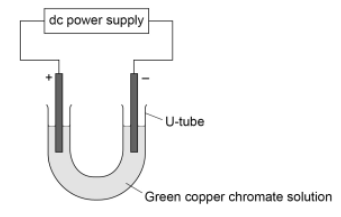
The student switched the power supply on. The student observed the changes at each electrode. Table 4 shows the student’s observations.
Table 4
Changes at positive electrode | Changes at negative electrode |
Solution turned yellow | Solution turned blue |
Bubbles formed at electrode | Solid formed on the electrode |
Describe how the solid forms at the negative electrode.
[3 marks]
(Source: AQA GCSE Chemistry Paper (Higher) 1 June 2020, Q4 (opens in a new tab))
Why this is hard:
Students know “copper forms at the negative electrode” but struggle to express oxidation and reduction accurately. Many write that “copper ions are deposited” or forget to mention the gain of two electrons, which is essential for full marks.
How to answer it:
Identify that Cu2+ ions move to the negative electrode.
State that they gain two electrons (reduction).
State that this forms copper atoms, which form the solid.
Model answer:
Cu2+ ions gain two electrons at the negative electrode to form copper atoms.
These copper atoms build up as a solid on the electrode.
Top tip:
Make sure you make the distinction between atoms (neutral particles) and ions (charged particles) very clear.
Positive ions become atoms when they gain electrons.
Negative ions become atoms when they lose electrons.
Energy Changes
Bond-energy questions test both your chemistry knowledge and your calculation skills. This example shows how to approach them methodically and avoid common mistakes.
Example Question:
Calculate the energy change for the following reaction using the bond energies provided:
CH4 + 2O2 → CO2 + 2H2O
Bond energies (kJ/mol): C–H = 412, O=O = 496, C=O = 805, O–H = 463
Is this reaction exothermic or endothermic?
[5 marks]
Why this is hard:
You need to correctly identify all bonds broken and formed, handle the stoichiometry, and remember that energy in and energy out have opposite signs.
How to answer it:
Calculate the energy required to break the bonds
Calculate the energy associated with forming the bonds
Calculate the difference
Combustion is an exothermic reaction that releases energy to the surroundings, so the value is negative
Model answer:
Step 1: Bonds broken (energy IN—positive)
CH4 has 4 C–H bonds: 4 × 412 = 1648 kJ
2O2 molecules have 2 O=O bonds: 2 × 496 = 992 kJ
Total energy in = 1648 + 992 = 2640 kJ
Step 2: Bonds formed (energy OUT—negative)
CO2 has 2 C=O bonds: 2 × 805 = 1610 kJ
2H2O have 4 O–H bonds: 4 × 463 = 1852 kJ
Total energy out = 1610 + 1852 = 3462 kJ
Step 3: Overall energy change
Energy change = Energy in – Energy out
2640 – 3462 = –822 kJ
The negative value means energy is released, so the reaction is exothermic.
Top tip:
Draw out the molecules if it helps you count bonds.
Remember: bonds broken = energy needed (positive), bonds formed = energy released (negative).
Rates of Reaction
Evaluation questions ask you to judge the quality of a method and suggest improvements. This example helps you practise spotting strengths, weaknesses, and realistic fixes.
Example Question:
A student investigates how temperature affects the rate of reaction between sodium thiosulfate and hydrochloric acid by timing how long it takes for a cross placed under the flask to disappear. Evaluate the student's method and suggest improvements.
[6 marks]
Why this is hard:
This is an evaluation question, you need to identify strengths and weaknesses, then suggest specific improvements. It tests your understanding of fair testing and accuracy.
How to answer it:
Think about variables, measurement, and reliability.
Strengths:
The disappearing cross is a clear end point
Easy to measure with a stopwatch
The method tests the variable (temperature) by keeping others constant
Weaknesses:
The end point is subjective (people judge when the cross disappears differently)
Difficult to ensure the temperature stays constant during the reaction
Lighting conditions could affect visibility
Only measures one rate (disappearance time)—doesn't track the full reaction
Improvements:
Use a light sensor to detect when the cross disappears objectively
Heat the sodium thiosulfate solution to the target temperature before mixing
Repeat the experiment and calculate a mean to improve reliability
Use a water bath to maintain constant temperature
Model answer:
The method is suitable because timing the disappearance of a cross gives a measurable end point. However, the method is subjective as different people may judge when the cross has disappeared differently, making results less reliable.
The student could improve this by using a light sensor connected to a data logger to record when a specific light level is reached. To ensure temperature is controlled, the student should heat the sodium thiosulfate in a water bath before adding the acid.
The student should also repeat the experiment at each temperature at least three times and calculate a mean to identify anomalies and improve accuracy. Remember that any anomalies should not be included in a calculation.
Top tip:
For evaluation questions, use the words "however" and "alternatively" to show you're considering different angles.
Chemical Equilibria
Equilibrium questions often require you to apply a rule and explain your reasoning clearly. This example shows how to break the question into simple steps so your answer stays focused.
Example Question:
The Haber process produces ammonia:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g) ΔH = –92 kJ/mol
A chemist increases the temperature of the reaction. Explain the effect this will have on the yield of ammonia and the rate of reaction. Use Le Chatelier's Principle in your answer.
[5 marks]
Why this is hard:
You need to apply Le Chatelier's Principle correctly and discuss the rate separately. Students often confuse yield with rate.
How to answer it:
Identify the reaction type.
ΔH is negative, so the forward reaction is exothermic.
Apply Le Chatelier's Principle for yield.
Increasing temperature favours the endothermic direction (the reverse reaction).
This means the equilibrium shifts to the left, producing less ammonia.
So, the yield decreases.
Now consider the rate.
Higher temperature means particles have more kinetic energy.
They collide more frequently and with more energy
So, the rate of reaction increases for both forward and backward reactions.
Model answer:
The forward reaction is exothermic (ΔH is negative).
According to Le Chatelier's Principle, increasing temperature favours the endothermic reverse reaction to oppose the change. This shifts the equilibrium position to the left, so the yield of ammonia decreases.
However, the rate of reaction increases because particles have more kinetic energy at higher temperatures. They collide more frequently and with more energy above the activation energy, so more successful collisions occur per second.
Top tip:
Always mention the direction of equilibrium shift and why.
Remember: rate and yield are different things!
Extended response questions
Extended-response questions, often worth 6 marks, are the 'mini-essay' questions of your exam. They aren't just testing what you know; they're testing how well you can communicate your understanding in a clear, logical argument.
Unlike other questions where you get a mark for each correct point, these are usually marked using a 'Level of Response' scheme. This means the examiner reads your entire answer and decides which level it fits into:
Level 1 = 1 - 2 marks
Level 2 = 3 - 4 marks
Level 3 = 5 - 6 marks
To reach the top level, your answer must:
Contain the right scientific facts
Be well-structured and coherent
Use precise scientific language throughout.
Simply listing a few keywords won't be enough; you need to build a proper scientific explanation.
Example Question:
This example shows how to organise your thoughts to build a top-level answer and earn those marks confidently.
Ethanol is manufactured by reacting ethene, C2H4, with steam.
The reaction is reversible and occurs in a closed system.
C2H4 (g) + H2O (g) ⇌ C2H5OH (g) ΔH = −45 kJ mol−1
Only 5% of the ethene is converted into ethanol at each pass through the reactor.
By removing the ethanol from the equilibrium mixture and recycling the unreacted ethene, it is possible to achieve an overall 95% conversion.
The diagram shows the conditions used:
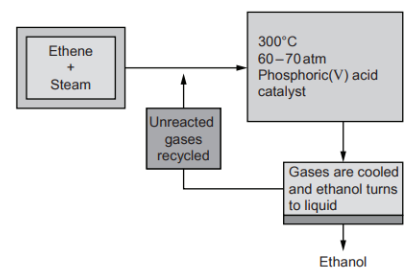
Explain why the conditions used for the process are chosen.
[6 marks]
(Source: GCSE (9–1) Chemistry A (Gateway Science) Paper 4 2018, Q19 (opens in a new tab))
Why this is hard:
It’s a Level of Response question. The examiner wants a structured, multi-point explanation, not a list of facts. You need to:
Apply Le Chatelier’s Principle
Explain the role of temperature, pressure, and the catalyst
Link each condition to yield and rate.
Many answers only mention one factor and lose marks.
How to answer it:
Recognise that the forward reaction is exothermic (ΔH is negative).
Explain how moderate temperature balances rate and yield.
Explain how high pressure increases yield (fewer gas moles on the right).
Mention that a catalyst increases rate, not yield.
Use clear reasoning: “because”, “so”, “this shifts the equilibrium…”.
Model answer:
Temperature: A temperature of around 300°C is used because lower temperatures give a higher yield for an exothermic reaction. But, lower temperatures make the rate of reaction too slow.
Pressure: Increasing the pressure shifts the equilibrium to the right because there are fewer moles of gas in the products. So, the yield of ethanol increases.
Catalyst: The phosphoric acid catalyst speeds up the reaction by lowering the activation energy. This gives a faster rate of reaction without changing the equilibrium position.
Top tip:
Most GCSE 6 mark questions aren't truly 6 markers, they're a linked set of 2 x 3-marks or 3 x 2 marks
This example question is question is 3 x 2 marks:
Temperature [2 marks]
Pressure [2 marks]
Catalyst [2 marks]
Exam Tips
Want to know the secrets that Chemistry teachers share with their top students? Here they are:
Learn command words inside out
Make flashcards for each one.
"Explain" needs reasoning.
"Describe" needs observation.
"Evaluate" needs judgement.
Get these right and you'll immediately gain marks
Practice with real past papers
There's no substitute for the actual exam experience. Time yourself, mark your work honestly, and learn from your mistakes
Create a formula sheet
Write down every equation you need to know (moles, concentration, energy, etc.) with examples. To make sure you've covered all the content, you can use our complete GCSE Chemistry Topics by Exam Board: Full List article as your ultimate checklist
Stick it somewhere you'll see it daily
Don't skip the "easy" marks
State symbols, units, and showing working might seem trivial, but they add up. Many students lose 10+ marks across a paper just from missing these details.
Revise definitions word-perfectly
Questions like "What is meant by activation energy?" need precise answers. Close enough doesn't cut it for definition marks.
Make friends with unfamiliar contexts
When you see a question about a weird scenario you've never studied, don't panic. Identify what chemistry you do know and apply it. That's exactly what the examiner wants to see.
Common Mistakes Students Make (and How to Avoid Them)
Let's talk about the things that trip students up:
Not reading the question properly
Always read carefully and answer what's actually being asked.
For example, you see "electrolysis" and immediately write everything you know about electrolysis, but the question asked specifically about the negative electrode.
Confusing similar terms
One wrong word can cost you marks. Make a list of commonly confused terms and learn the differences. For example:
Exothermic vs endothermic
Oxidation vs reduction
Atom vs ion
Chlorine vs chloride
Rounding too early
If you round in step 2 of a 5-step calculation, your final answer may be wrong. Keep full calculator numbers until the very end, then round appropriately (usually 2–3 significant figures).
Not showing working
Imagine that you have a calculation question:
You do the calculation in your head.
You only write the final answer.
But, you made a small / simple mistake…
Zero marks.
Always show your method, even for "easy" questions.
Forgetting to balance equations
You know the products, but your equation has different numbers of atoms on each side. Check your balancing before moving on. Remember that this could have knock on effects for subsequent calculations.
Panicking when the context is unfamiliar
Imagine that you are given a question about extracting chromium. Your first thought might be "I've never heard of chromium extraction!"
Don't worry, you're not supposed to have. The examiner is testing if you can apply your knowledge of extraction to a new metal. Break it down to basics.
Writing too much (or too little)
A 2-mark question needs 2 clear points, not a paragraph. A 6-mark question needs detailed explanation, not two sentences. Use the marks as your guide.
Frequently Asked Questions
What Topics Have the Hardest Questions in GCSE Chemistry?
The toughest questions usually appear in a few predictable topics:
Quantitative Chemistry (moles, calculations)
Chemical Equilibria (Le Chatelier's principle)
Electrolysis
By focusing your revision on mastering the rules and formulas for these areas, you'll be well-prepared for the most challenging parts of the paper.
How Do I Improve My Confidence With Hard Chemistry Questions?
Practice is everything.
Start with easier questions to build your foundation, then gradually work up to the tough ones.
Use mark schemes actively, don't just check if you're right; study how the examiner wants the answer phrased.
Work through questions before looking at the answer.
It's tempting to read the solution first, but that doesn't build problem-solving skills.
Struggle a bit, try different approaches, then check your work.
Learn from your mistakes.
Keep a "mistakes book" where you write down questions you got wrong.
Add notes about why you got them wrong and how to get them right next time.
Review this regularly.
How Many Marks Are the Hardest Questions Usually Worth?
The trickiest questions are typically worth 4 - 6 marks and appear towards the end of each section or paper. These questions test multiple skills at once and often require extended answers.
Six-mark questions are special, they're usually "extended response" questions that test your ability to write coherently, link ideas, and use scientific terminology accurately. You'll need to structure your answer logically, like a mini-essay.
However, don't assume a 1-mark question is automatically easy. Sometimes a single-mark question asks for a precise definition or a specific term that you either know or don't. These can be just as challenging as multi-mark questions if you haven't revised thoroughly.
Final Thoughts
Here's the truth: mastering the hardest GCSE Chemistry questions isn't about being naturally gifted at science. It's about:
Learning how to think like an examiner.
Practising the tricky bits over and over.
Building your confidence one question at a time.
To take your preparation to the next level, discover the key strategies in our guide on How to Get a Grade 9 in GCSE Chemistry.
The most effective approach to revision really does vary from student to student, so it’s worth experimenting until you find what suits you best. For more detailed strategies on planning your time and using active recall, read our complete guide on How to Revise for Chemistry GCSE.
In my experience, the strongest revision plans combine different types of resources. For example:
Use your class notes, our revision notes and textbooks to build understanding.
Use past papers to practise exam technique.
Use flashcards for key definitions.
Watch videos and use diagrams to bring abstract processes to life.
Mixing these methods keeps revision fresh and helps you strengthen both your memory and your problem-solving skills.
The Save My Exams GCSE Chemistry library contains all the resources you need:
References:
AQA GCSE Chemistry (opens in a new tab)
OCR GCSE Chemistry (opens in a new tab)
Edexcel GCSE Chemistry (opens in a new tab)
AQA GCSE Chemistry Paper (Higher) 1 June 2023 (opens in a new tab)
AQA GCSE Chemistry Paper 1 2023 Mark Scheme (opens in a new tab)
GCSE (9–1) Chemistry A (Gateway Science) Paper 4 2018 (opens in a new tab)
GCSE (9–1) Chemistry A (Gateway Science) Paper 4 2018 Mark Scheme (opens in a new tab)
GCSE (9–1) Chemistry A (Gateway Science) Paper 4 2018 Examiner Report (opens in a new tab)
AQA GCSE Chemistry Paper (Higher) Paper 1 June 2022 Mark Scheme (opens in a new tab)
AQA GCSE Chemistry Paper (Higher) 1 June 2020 (opens in a new tab)
AQA GCSE Chemistry Paper (Higher) 1 June 2020 Mark Scheme (opens in a new tab)
AQA GCSE Chemistry Paper (Higher) 1 Examiner Report 2020 (opens in a new tab)
Was this article helpful?
Sign up for articles sent directly to your inbox
Receive news, articles and guides directly from our team of experts.

Share this article
 written revision resources that improve your
written revision resources that improve your