Practical: Energy Content of Food (SQA National 5 Biology): Revision Note
Exam code: X807 75
Investigating the energy content of food
We can investigate the energy content of food in a simple calorimetry experiment
This involved burning a sample of food under a known volume of water
The energy from the food is transferred into the heat of the flames, which in turn is transferred into the heat of the water
The change in temperature of the water sample reflects the quantity of energy in the sample and can be calculated mathematically
Examiner Tips and Tricks
Note that this practical is a 'suggested practical' in the specification, rather than content that all students are expected to learn. Some schools may choose to complete alternative practicals, or may miss out practical work that is not realistic, e.g. due to equipment or time constraints.
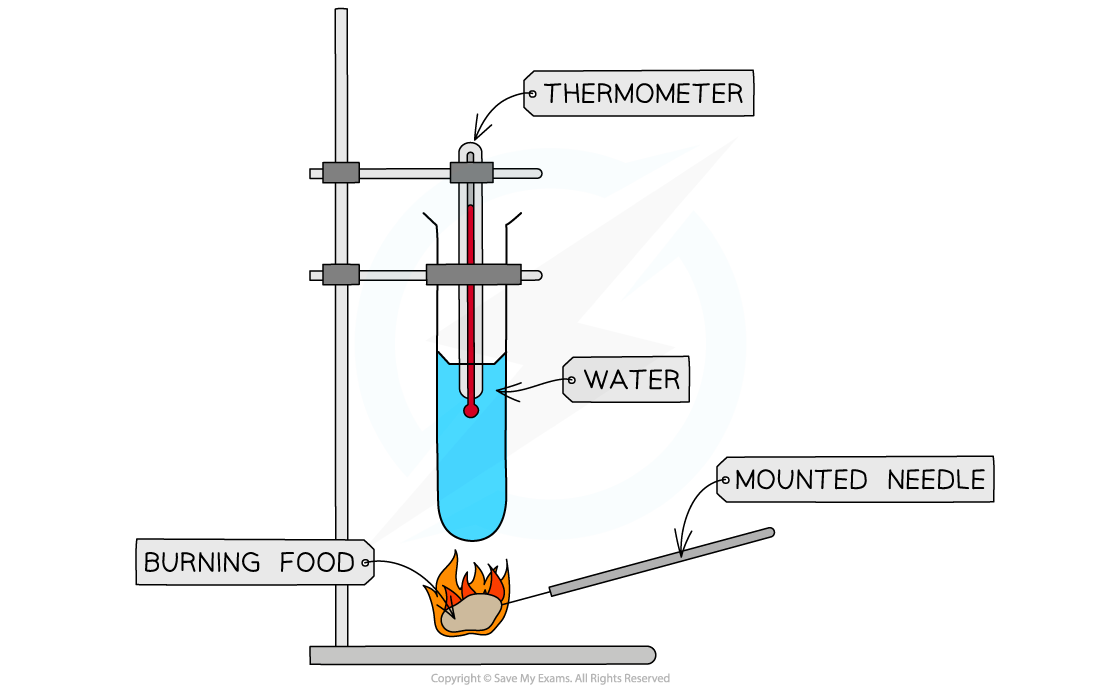
Apparatus
Boiling tube
Boiling tube holder
Bunsen burner
Mounted needle
Measuring cylinder
Balance/scales
Thermometer
Water
Food samples
Method
Use the measuring cylinder to measure out 25cm3 of water and pour it into the boiling tube
Record the starting temperature of the water using the thermometer
Weigh the initial mass of the food sample
Set fire to the sample of food using the Bunsen burner and hold the sample 2cm from the boiling tube until it has completely burned
Record the final temperature of the water
Repeat the process with different food samples
e.g. popcorn, nuts, crisps
Expected results
The larger the increase in water temperature, the more energy is stored in the sample
We can calculate the energy in each food sample using the following equation:
4.2 kJ is the specific heat capacity of water
1 cm3 of water has a mass of 1 g
Food sample | Mass of water / g | Mass of food / g | Initial water temperature / °C | Final water temperature / °C | Change in water temperature / °C | Energy transferred per gram of food (J) |
|---|---|---|---|---|---|---|
Popcorn | 25 | 8.5 | 20.5 | 31.2 | 10.7 | 132.2 |
Walnut | 25 | 8.1 | 20.4 | 34.1 | 13.7 | 177.6 |
Limitations
Incomplete burning of the food sample
Solution: Relight the food sample until it no longer lights up
Heat energy is lost to the surroundings
Solution: Whilst heat lost means that the energy calculation is not very accurate, so long as the procedure is carried out in the same way each time (with the same distance between the food sample and boiling tube), we can still compare the results

Unlock more, it's free!
Was this revision note helpful?
