Antibodies (Cambridge (CIE) A Level Biology): Revision Note
Exam code: 9700
Antibodies: structure & functions
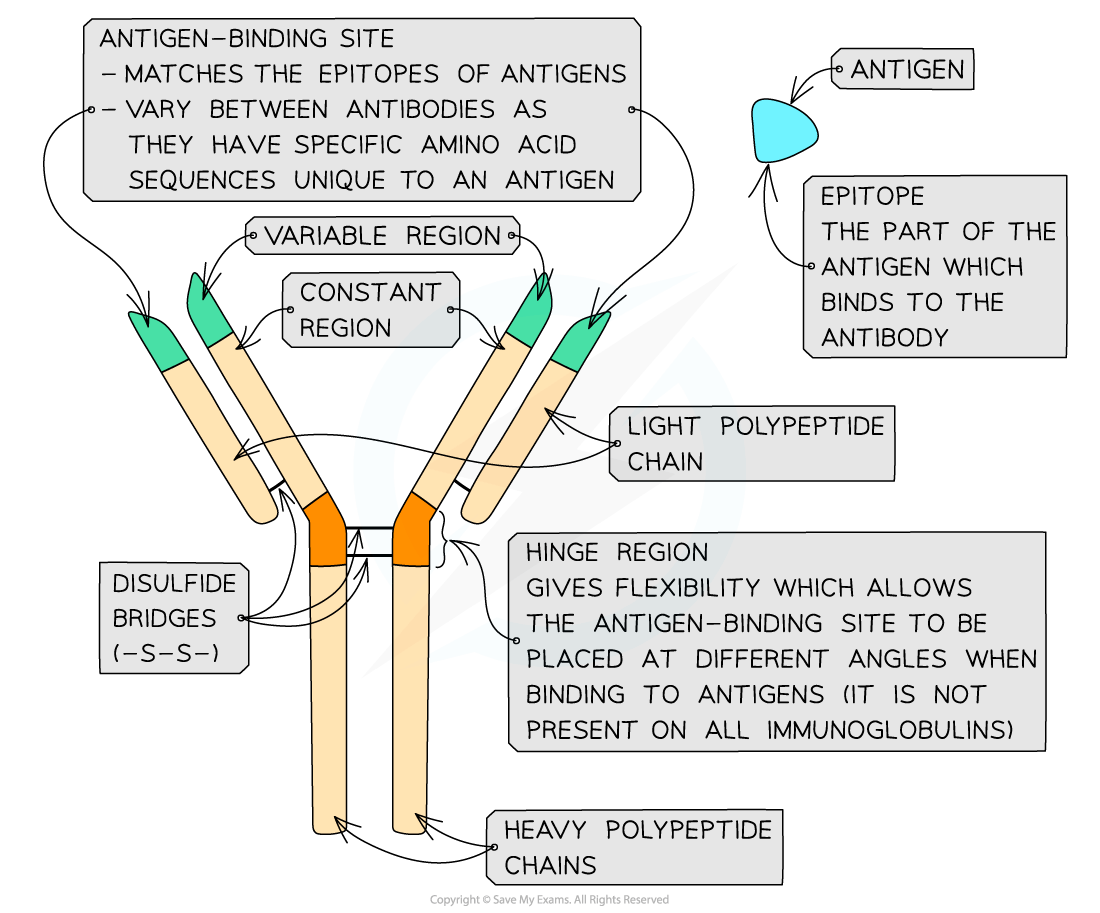
Antibody structure
Antibodies are globular glycoproteins called immunoglobulins
Antibodies have a Y-shaped quaternary structure with two ‘heavy’ polypeptide chains bonded by disulfide bonds to two ‘light’ polypeptide chains
Antibodies have a constant region and a variable region
The constant regions do not vary within a class of antibodies but do vary between the classes.
The constant region determines the mechanism used to destroy antigens
The variable region is where the antibody attaches to the antigen to form an antigen-antibody complex
At the end of the variable region is the antigen-binding site.
Each antigen-binding site is generally composed of 110 to 130 amino acids and includes the ends of the light and heavy chains
Antigen-binding sites vary greatly, giving the antibody its specificity for binding to antigens
Antibodies bind to a region of the antigen called the epitope
Antibodies also have a hinge region which gives flexibility to the antibody molecule
This allows the antigen-binding site to be placed at different angles when binding to antigens
Antibody function
Antibodies are produced by B lymphocytes
Their role is to bind to specific antigens
Antigens include parts of pathogens and their toxins, pollen, blood cell surface molecules and the surface proteins found on transplanted tissues
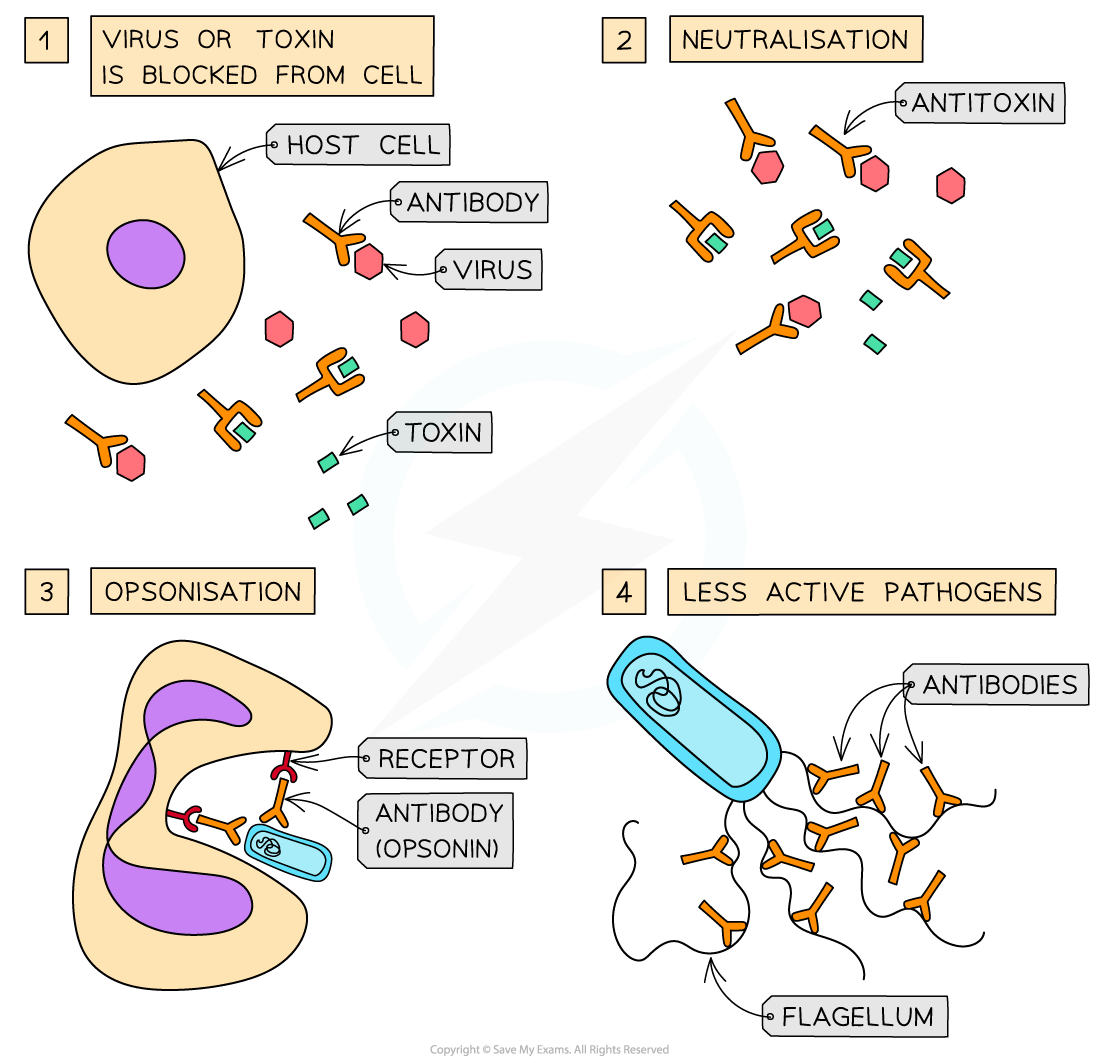
Antibodies can function in several different ways
Antibodies can attach to viruses and to the toxins produced by pathogens to block them from entering or damaging cells
Antibodies can act as anti-toxins by binding to and neutralising toxins
Antibodies can attach to bacteria, making them readily identifiable to phagocytes; this is called opsonisation.
Once identified, the phagocyte has receptor proteins for the heavy polypeptide chains of the antibodies, which enables phagocytosis to occur
Antibodies can attach to the flagella of bacteria, making them less active; this makes it easier for phagocytes to do phagocytosis
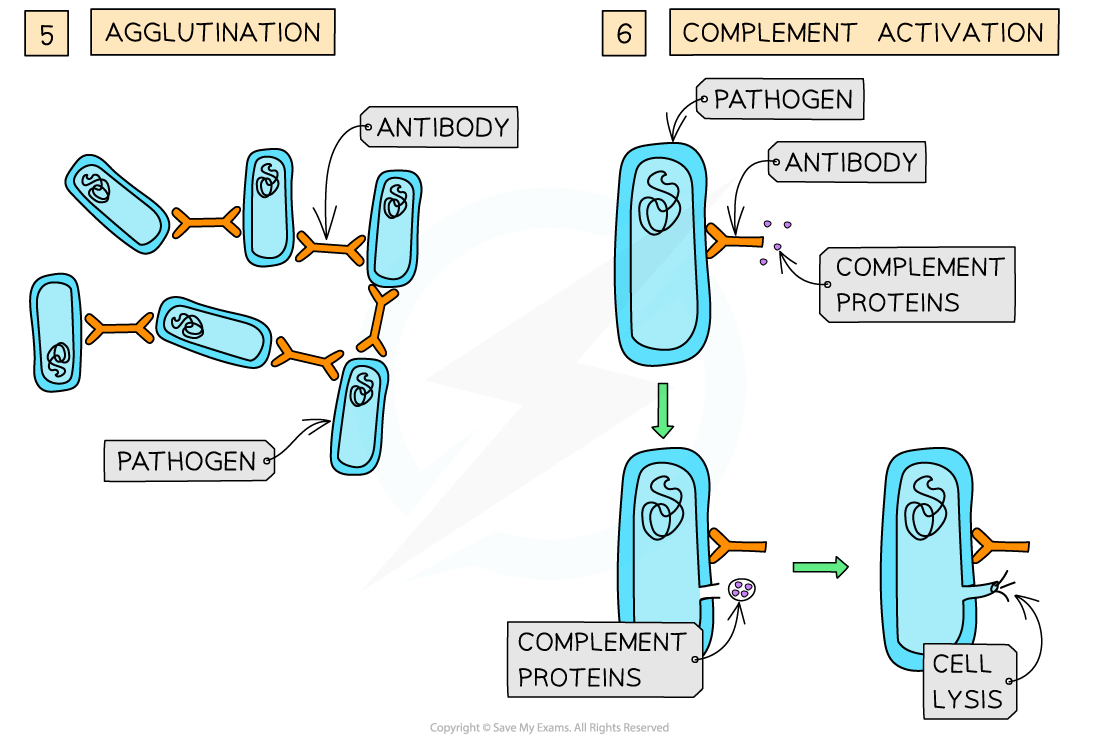
Antibodies act as agglutinins, causing pathogens to clump together in agglutination
This reduces the chance that the pathogens will spread through the body and makes it possible for phagocytes to engulf a number of pathogens at one time
Antibodies can create holes in the cell walls of pathogens causing them to burst, or lyse, when water is absorbed by osmosis

Unlock more, it's free!
Was this revision note helpful?
