Water & the Hydrogen Bond (Cambridge (CIE) A Level Biology): Revision Note
Exam code: 9700
Water molecules: hydrogen bonds
Water is of great biological importance. It is the medium in which all metabolic reactions take place in cells
Between 70% to 95% of the mass of a cell is water
As 71% of the Earth’s surface is covered in water it is a major habitat for organisms
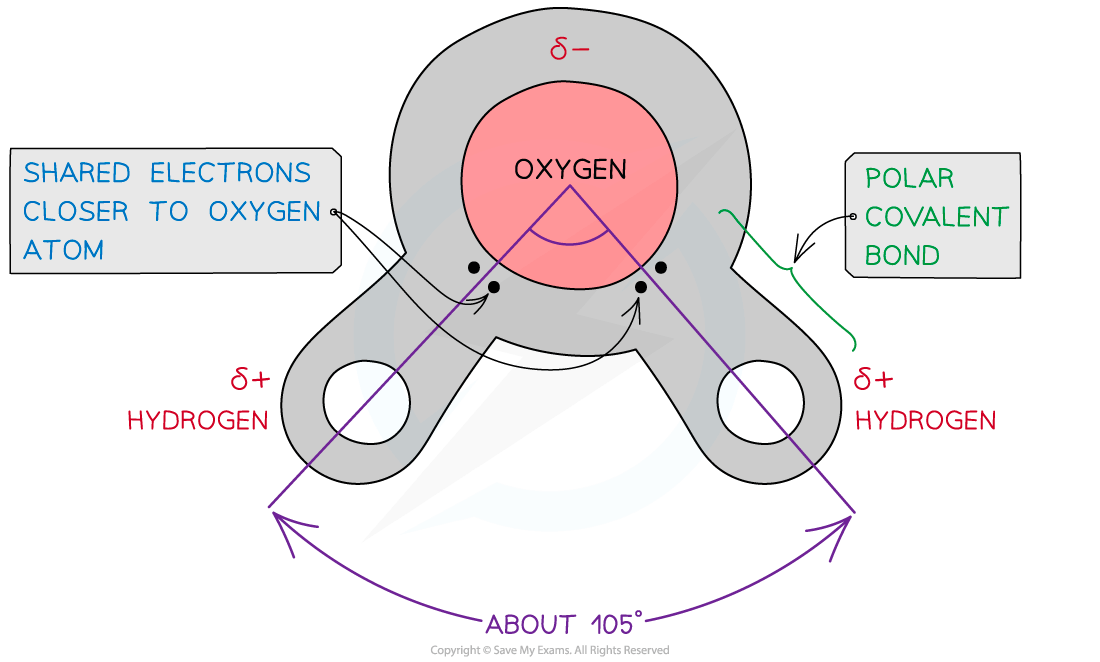
Water is composed of atoms of hydrogen and oxygen
One atom of oxygen combines with two atoms of hydrogen by sharing electrons so they are covalently bonded
Although water as a whole is electrically neutral the sharing of the electrons is uneven between the oxygen and hydrogen atoms
The oxygen atom attracts the electrons more strongly than the hydrogen atoms, resulting in:
A weak negatively charged region on the oxygen atom (δ-)
A weak positively charged region on the hydrogen atoms(δ+)
This results in the asymmetrical shape of water molecules
This separation of charge due to the electrons in the covalent bonds being unevenly shared is called a dipole
When a molecule has one end that is negatively charged and one end that is positively charged it is also a polar molecule
Water is a polar molecule
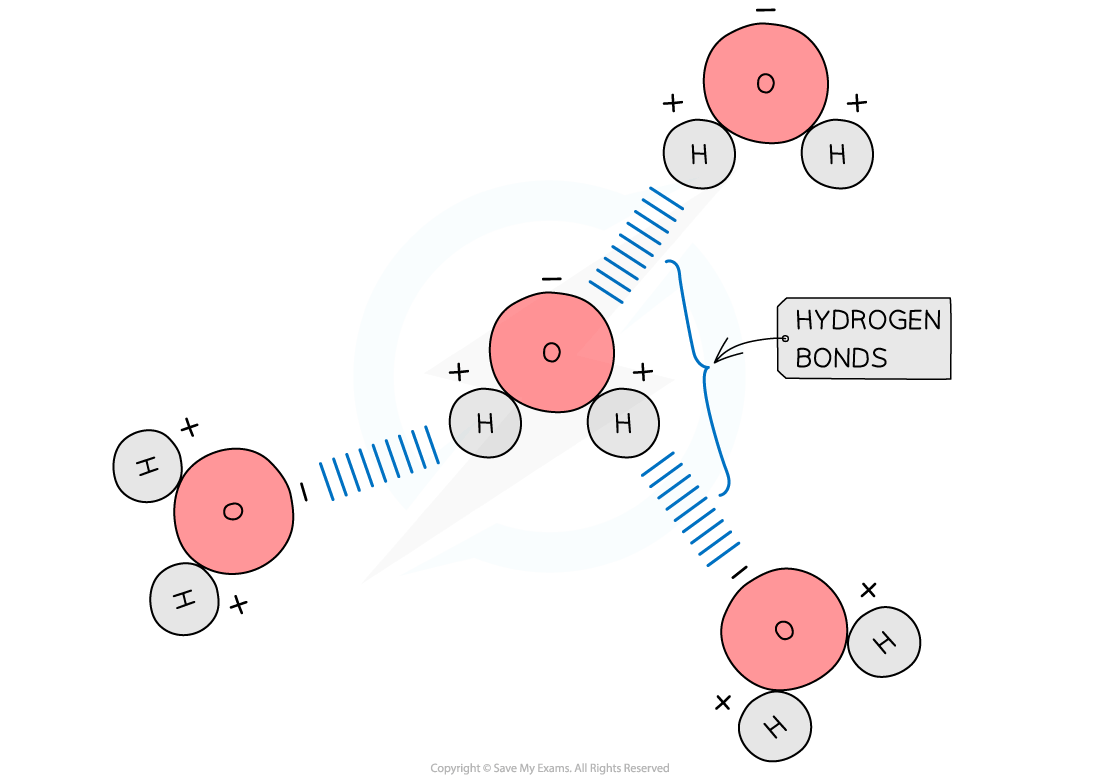
Hydrogen bonds form between water molecules
As a result of the polarity of water hydrogen bonds form between the positive and negatively charged regions of adjacent water molecules
Hydrogen bonds are weak, when there are few, so they are constantly breaking and reforming
However when there are large numbers present they form a strong structure
Hydrogen bonds contribute to the many properties water molecules have that make them so important to living organisms:
An excellent solvent – many substances can dissolve in water
A relatively high specific heat capacity
A relatively high latent heat of vaporisation
Water is less dense when a solid
Water has high surface tension and cohesion
It acts as a reagent
Examiner Tips and Tricks
It is important to know where the hydrogen bonds form between water molecules (oxygen of one water molecule to the hydrogen atom of another).
Don't get confused with the covalent bonds between the atoms within a water molecule.

Unlock more, it's free!
Was this revision note helpful?
