Evaluating in Chemistry (DP IB Chemistry): Revision Note
Evaluating in Chemistry
The evaluation is a critical reflection on your investigation's methodology.
This is where you demonstrate your understanding of the scientific process by identifying the weaknesses and limitations of your own work.
The goal is to assess the quality of your data and its impact on your conclusion, and to suggest meaningful, realistic improvements.
Principles of evaluation
Evaluate your hypothesis
This is the final comment on your hypothesis, which should follow on from your conclusion.
Even if your data supported your hypothesis, you should evaluate the strength of this support in light of the uncertainties and errors you have identified.
For example:
The data supported the hypothesis that the reaction is first-order.
But, the significant scatter of data points around the line of best fit suggests that random errors had an impact.
Therefore, the support for the hypothesis is not as strong as it could be.
Identify and discuss sources of error
This is the most important part of your evaluation.
You must identify and discuss specific sources of error in your procedure, distinguishing between the two main types.
Systematic errors:
These are flaws in the experimental method or apparatus that cause the result to be consistently wrong in the same direction (e.g., always too high or always too low).
Example 1:
Significant heat loss to the surroundings in a calorimetry experiment.
This will always cause the measured temperature change to be smaller than the true value, making the calculated ΔH consistently less exothermic.
Example 2:
A poorly calibrated digital balance that always reads 0.2 g too high.
Repeating trials does not reduce systematic error.
Random errors:
These are unpredictable variations in measurements that occur by chance.
They cause results to be scattered around the true value.
Example 1:
Subjectivity and variations in reaction time when judging the endpoint of a titration by eye.
Example 2:
Fluctuations in reading a burette meniscus.
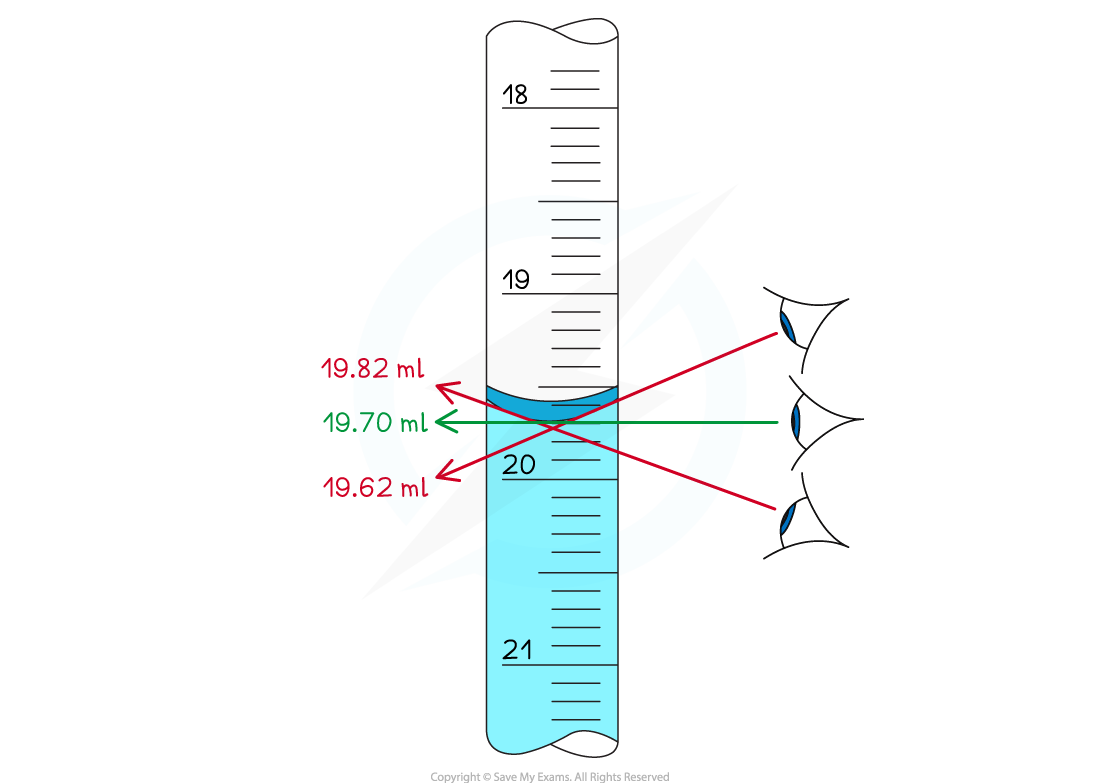
How random error occurs reading a meniscus.
Random errors can be minimised by taking multiple repeat trials and calculating an average.
Evaluate methodological weaknesses, limitations and assumptions
Beyond errors, you should also discuss other aspects of your method that affect the quality of your conclusion.
Weaknesses:
These are the aspects of your method that lead to significant systematic or random errors.
For example:
Using a glass beaker instead of a polystyrene cup for a calorimetry experiment is a key methodological weakness, as glass is a poor thermal insulator and allows for significant heat loss.
Limitations:
These are factors that limit the scope of your conclusion. They define the boundaries within which your conclusion is valid.
For example:
This investigation was limited to the first four primary alcohols.
Therefore, the conclusion that enthalpy of combustion increases linearly with chain length cannot be assumed to be valid for longer-chain alcohols.
Assumptions:
These are simplifications made during your calculations that are not perfectly true.
For example:
In the enthalpy of neutralisation calculation, it was assumed that the specific heat capacity and density of the aqueous solutions were the same as those of pure water.
This is a minor assumption that introduces a small inaccuracy into the final result.
Explain realistic and relevant improvements
For every significant weakness or source of error you identify, you must suggest a specific, realistic improvement.
The improvement must be relevant.
It should directly address the weakness you identified.
The improvement must be realistic.
You should be able to carry it out in a typical school laboratory.
For example, using a bomb calorimeter is not a realistic improvement.
Worked Example
Research question:
"What is the enthalpy of combustion of the primary alcohols (methanol to butan-1-ol)?"
Weakness 1 (systematic error):
The most significant weakness in the methodology was heat loss to the surroundings from the copper calorimeter.
Impact:
Heat produced by the burning alcohol was lost to the air instead of being transferred to the water.
This caused the measured temperature change (ΔT) to be consistently smaller than the true value
This resulted in a calculated enthalpy of combustion (ΔH) that was significantly less exothermic than the literature value for all four alcohols.
Realistic Improvement:
To reduce heat loss, the experiment should be repeated with an insulating lid on top of the calorimeter and a draught shield placed around the entire apparatus to minimise convection.
Weakness 2 (systematic/random error):
Incomplete combustion of the longer-chain alcohols.
Impact:
The qualitative observation of soot on the calorimeter for propan-1-ol and butan-1-ol indicates that not all the fuel was converted to CO2 and H2O.
Incomplete combustion releases less energy.
So, this contributed to the calculated ΔH values being less exothermic, especially for the larger alcohols.
Realistic Improvement:
The distance between the wick and the calorimeter could be adjusted systematically to find the optimal height for complete combustion, or a spirit burner with a wick that allows for oxygen control could be used.
Limitation:
The investigation was limited to the first four straight-chain primary alcohols.
Impact:
The conclusion of a linear relationship between chain length and ΔH is only supported within this limited range.
Realistic Improvement:
The investigation could be extended to include pentan-1-ol and isomers such as propan-2-ol to determine if the trend continues and how branching affects the enthalpy of combustion.
Examiner Tips and Tricks
Be specific.
Never blame "human error".
Instead of saying "My measurements were wrong," identify a specific source of error, like "The parallax error involved in reading the meniscus of the measuring cylinder could have led to inconsistent volume measurements."
Prioritise your evaluation.
Focus on the one or two most significant sources of error that had the biggest impact on your final result.
Discussing heat loss in a calorimetry lab is always more important than discussing the precision of the balance.
Close the loop: Weakness → Impact → Improvement.
For every weakness you identify, you must explain its impact on your final result and then suggest a specific improvement to fix it.
Evaluate your own data.
Do not write a generic evaluation that could apply to any experiment.
Refer back to your own results, graphs, and observations.
For example, "The large error bars at higher temperatures suggest that measuring the rapid reaction times was difficult, leading to a loss of precision."

Unlock more, it's free!
Was this revision note helpful?
