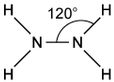
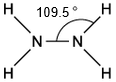
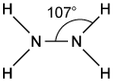
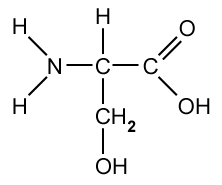
Silver and iodine are both shiny crystalline solids.
Which forces exist between neighbouring iodine molecules in solid iodine and particles in solid silver?
| iodine | silver |
A | metallic bonds | covalent bonds |
B | ionic bonds | metallic |
C | covalent bonds | covalent bonds |
D | London dispersion forces | metallic |
Was this exam question helpful?