Causes & Impacts of Ozone Depletion (Cambridge (CIE) IGCSE Environmental Management): Revision Note
Exam code: 0680
What causes ozone depletion?
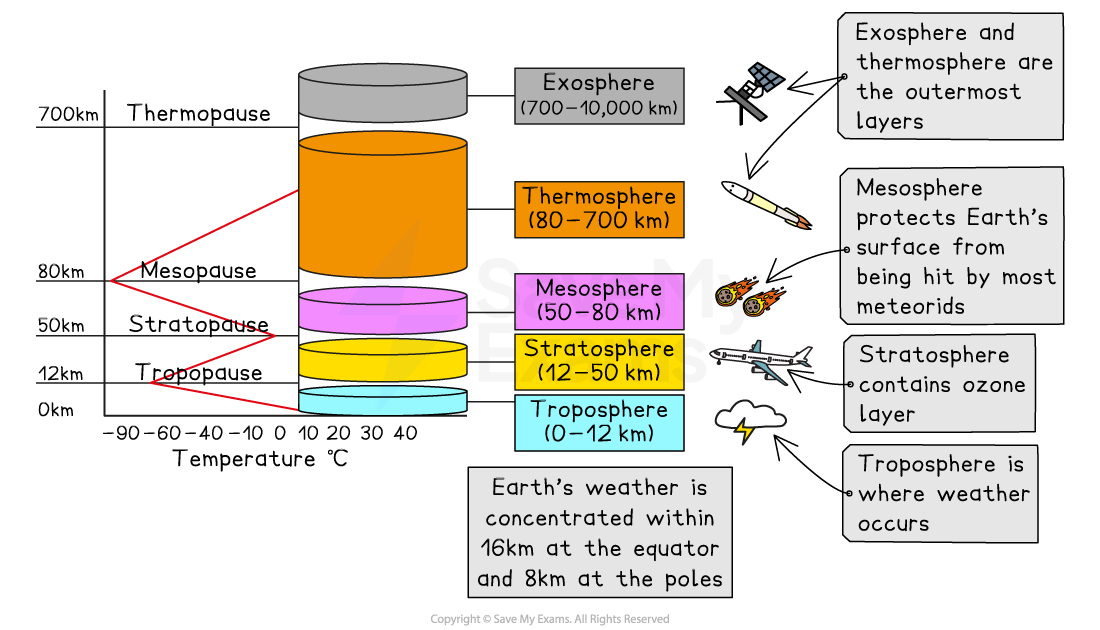
The stratosphere is the second layer of Earth's atmosphere and extends roughly 38 km (24 miles) between the troposphere and the mesosphere
The highest concentration of ozone gas is within the first 3-20 km of the lower stratosphere (15-30 km above the Earth's surface)
This layer acts as a natural 'sunscreen' for life on Earth by absorbing the majority of the sun's harmful ultraviolet (UV) radiation before it reaches the planet's surface
Stratospheric ozone depletion
Some substances break down ozone even more than natural levels
These chemicals, known as ozone-depleting substances (ODSs), lower the amount of ozone in the stratosphere
The main ODS gases are hydrogen and nitrogen oxides and those containing chlorine
One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere
Human-produced ODSs
Human-produced ODSs include
Aerosols containing chlorofluorocarbons (CFCs), such as sprays, foams, plastic manufacturing, deodorants and more
When released into the atmosphere during spraying, these substances can eventually reach the stratosphere and contribute to ozone depletion
Refrigerants in cooling systems, such as air conditioners and refrigerators, can leak and reach the stratosphere to contribute to ozone depletion
Even though ODSs are produced and emitted at the Earth's surface, it can take from two to five years for the substances to reach and affect the stratosphere
Natural ODSs
Natural processes, such as large volcanic eruptions, have an indirect effect on ozone levels
Mt. Pinatubo's 1991 eruption produced large amounts of aerosols (small particles) that increased chlorine's effectiveness at destroying ozone, although the effect was short-lived
Polar stratospheric clouds (PSCs) form during the long, cold Antarctic winter
These clouds consist of tiny ice crystals in the stratosphere
Chlorine compounds, mainly man-made CFCs, are trapped within the ice crystals, forming chlorine reservoirs
During the Antarctic spring, the returning sunlight melts the ice crystals, releasing and reactivating the chlorine atoms
This causes rapid stratospheric ozone depletion and the formation of the 'ozone hole' over Antarctica
Not all chlorine sources cause ozone layer depletion
Chlorine from pools, factories, sea salt, and volcanoes does not reach the stratosphere
Because ODSs are stable and do not dissolve in rain, there are no natural processes that remove them from the lower atmosphere
Examiner Tips and Tricks
Do not confuse global warming with ozone depletion.
Know the differences in causes and effects.
Ozone depletion is the thinning of the ozone layer and global warming is the raising of atmospheric temperatures.
Although the ozone layer is thinner, it does not allow increased levels of heat into the atmosphere, only ultraviolet light.
Impacts of ozone depletion
Ozone depletion refers to the gradual thinning of the ozone layer in the Earth's stratosphere
Stratospheric ozone depletion leads to increased levels of harmful ultraviolet (UV) radiation reaching the Earth's surface
This negatively impacts human health, including
Higher UV-B radiation exposure significantly increases the risk of skin cancer, particularly melanoma, the most dangerous form
Increased UV radiation can lead to the development of cataracts, clouding of the lens in the eye
Excessive UV exposure can weaken the immune system, making individuals more susceptible to infections
Chronic exposure to UV radiation accelerates the ageing process of the skin, causing the breakdown of collagen and elastin fibres, leading to wrinkles, sagging skin and the development of age spots
Sunburn occurs when the skin is exposed to excessive UV rays that trigger an inflammatory response as a defence mechanism, indicating damage to the skin cells
Ecosystems are damaged, affecting productivity
UV radiation can harm phytoplankton, the base of the marine food chain, impacting aquatic ecosystems
High UV levels can damage plant tissues, affecting crop yields and plant productivity
Disruptions in ecosystems due to UV damage can affect various species and alter food webs
Clothing and construction materials exposed to UV rays are damaged and degraded
Fabrics, plastics, paints and building materials become brittle, faded or weakened, reducing their durability and lifespan
This reduces the material's structure and function and also its visual appeal
Examiner Tips and Tricks
Don't confuse ozone depletion with the ozone hole.
Ozone depletion affects the entire Earth's stratosphere.
The ozone hole is an area of very low stratospheric ozone.
The hole is primarily over Antarctica in spring but can extend to South America and the Falkland Islands. The size and location of the ozone hole change depending on seasonal conditions.

Unlock more, it's free!
Was this revision note helpful?
