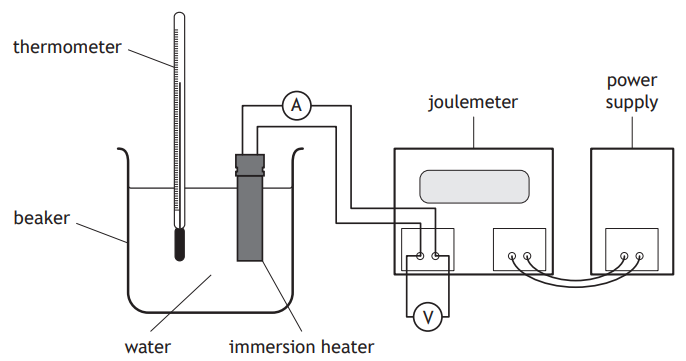
A student carries out an experiment to determine the specific heat capacity of copper using the apparatus shown.
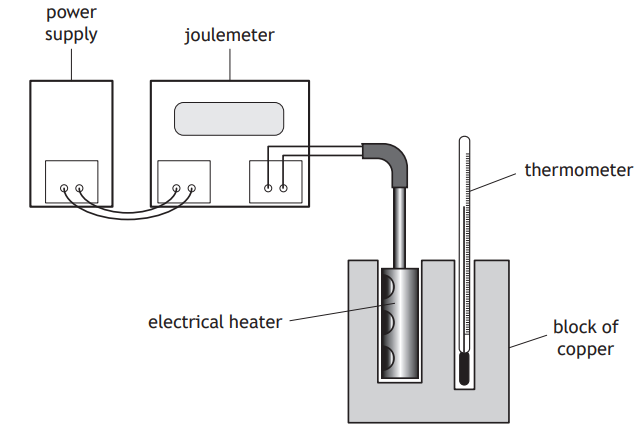
The student switches on the power supply and the electrical heater heats the block of copper.
The joulemeter measures the energy supplied to the electrical heater.
The student suggests the following measurements should also be made:
I The mass of the block of copper.
II The initial and final readings on the thermometer.
III The power rating of the electrical heater.
Which of these measurements must be made to determine the specific heat capacity of copper?
I only
II only
I and II only
II and III only
I, II and III
Was this exam question helpful?