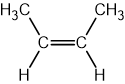
Name the two types of polymerisation.
Name two families of chemicals that can be used to form condensation polymers.
Name the two types of link formed by condensation polymerisation.
Name the two condensation products of the reaction between a dicarboxylic acid and a diamine.
Was this exam question helpful?