Gas Law Experiments (SQA National 5 Physics): Revision Note
Exam code: X857 75
Gas law experiments
The three gas laws can be verified by experiment
Exam questions may ask about these experiments in the following ways:
experimental verification
analysis and interpretation
suggested improvements
Verifying the relationship between pressure & volume
For a gas at constant temperature:
Pressure increases if the volume decreases
Pressure decreases if the volume increases
Pressure and volume are inversely proportional
Look for:
patterns in the data that show this relationship
a curved line on a graph that shows an inverse proportional relationship
The relationship between volume and pressure

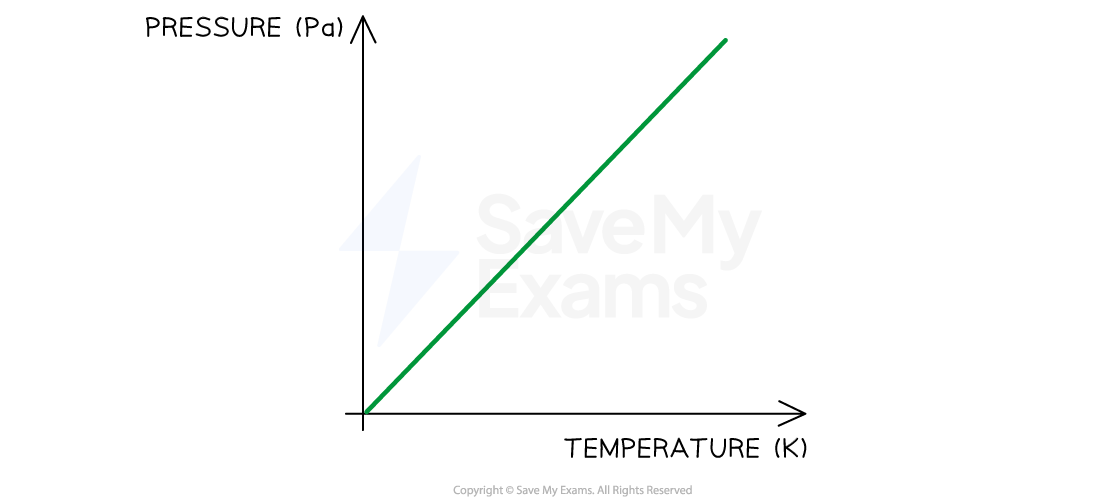
Verifying the relationship between pressure & temperature
For a gas at constant volume:
The pressure increases if the temperature increases
The pressure decreases if the temperature decreases
Pressure and temperature are directly proportional
Look for:
patterns in the data that show this relationship
a straight line that goes through the origin on a graph that shows a directly proportional relationship
The relationship between pressure and temperature
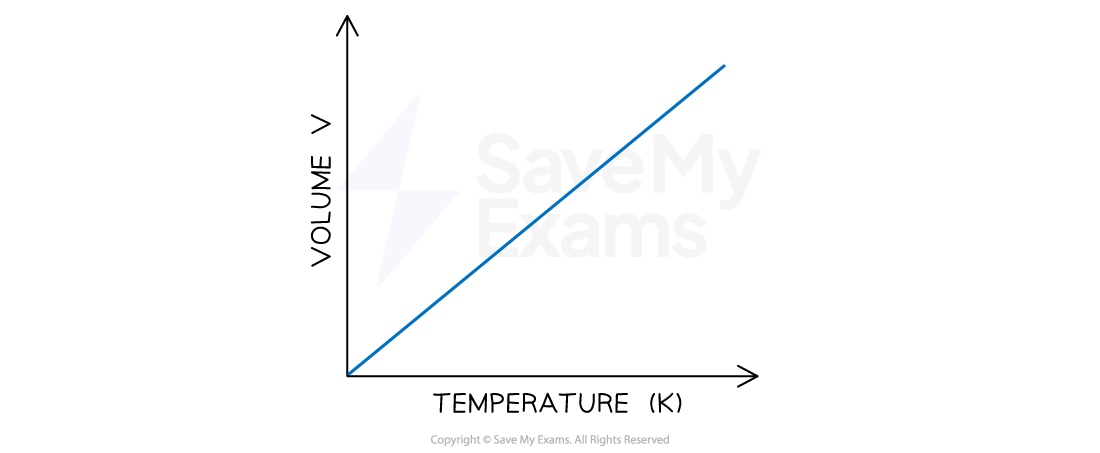
Verifying the relationship between volume & temperature
For a gas at constant pressure:
The volume will increase if the temperature increases
The volume will decrease if the temperature decreases
Volume and temperature are directly proportional
Look for:
patterns in the data that show this relationship
a straight line that goes through the origin on a graph that shows a directly proportional relationship
The relationship between volume and temperature
Worked Example
A group of students investigated the relationship between pressure and temperature.
They heated a round-bottomed flask containing a fixed mass of gas.
Readings of temperature and pressure were taken every 10 °C.
The student's results are shown.
Temperature (°C) | Pressure (kPa) | |
|---|---|---|
20 | 109.3 | |
30 | 113.0 | |
40 | 116.7 | |
50 | 120.4 | |
60 | 124.0 |
Use all the appropriate data to establish the relationship between the pressure and temperature of the gas.
Answer:
Step 1: Convert the temperatures into Kelvin
Use the empty column to add the values
Use the appropriate relationship to perform the conversion
Temperature (°C) | Temperature (K) | Pressure (kPa) |
|---|---|---|
20 | (20 + 273 =) 293 | 109 |
30 | (30 + 273 =) 303 | 113 |
40 | (40 + 273 =) 313 | 117 |
50 | (50 + 273 =) 323 | 120 |
60 | (60 + 273 =) 333 | 124 |
Step 2: Verify the relationship between temperature and pressure
State the relationship between temperature and pressure
Use the data to verify this relationship
Each set of readings gives a constant value of 0.37 (2 s.f.)
This proves the relationship:

Unlock more, it's free!
Was this revision note helpful?
