Relative Ionising Effect (SQA National 5 Physics): Revision Note
Exam code: X857 75
Relative ionising effect
Ionisation
Ionisation is when an atom becomes charged by gaining or losing electrons
When a neutral atom loses a negative electron, it becomes positively charged
When a neutral atom gains a negative electron, it becomes negatively charged
When an atom is ionised, it becomes an ion
Atoms are only called atoms when they are neutral
Nuclear radiation (
,
and
) can ionise atoms
This is mostly done by removing an electron, giving the ion an overall positive charge
Nuclear radiation ionising an atom
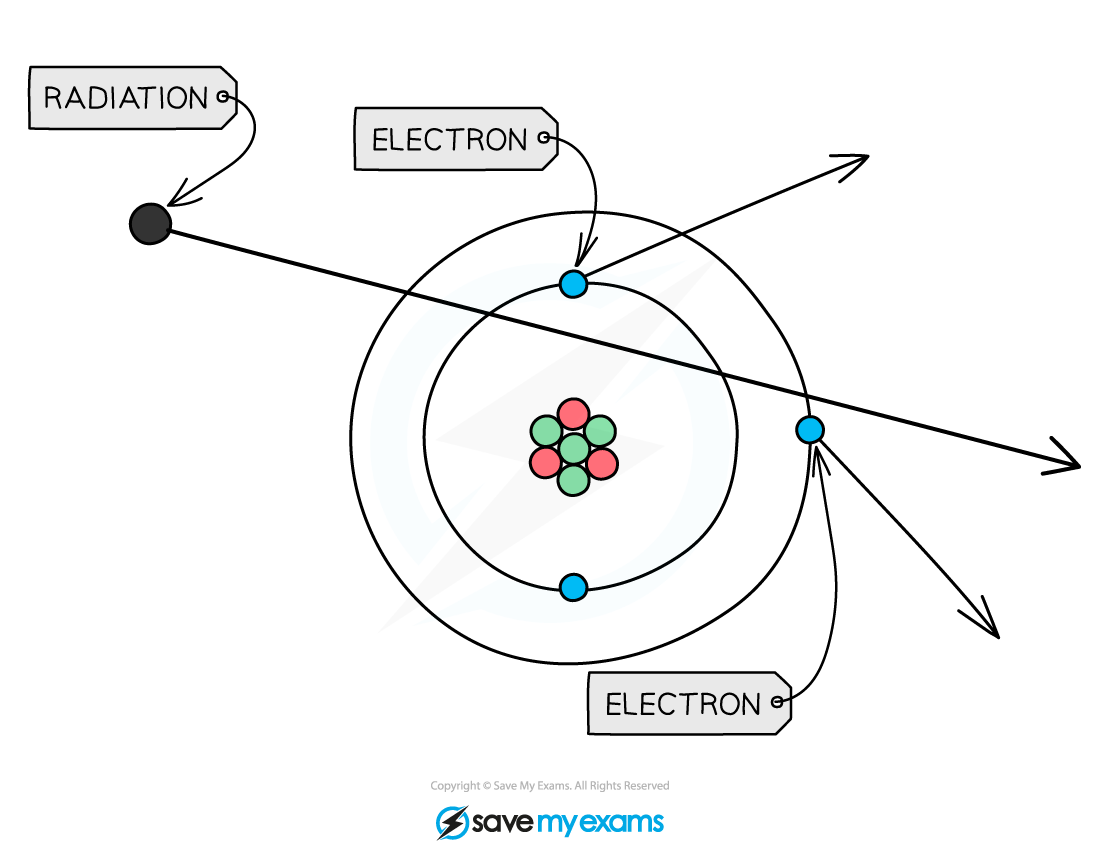
When radiation passes close to atoms it can knock out electrons, ionising the atom
α, β and γ radiation can be identified by the emission from a nucleus by recalling their:
Nature (what type of particle or radiation they are)
Their relative ionising effects (how easily they ionise other atoms)
Their relative penetrating abilities (how far can they travel before they are stopped completely)
The properties of alpha, beta and gamma are given in the table which shows the following trends down the table:
The range increases
Penetrating power increases
Ionisation decreases
Summary of the properties of nuclear radiation
Particle | Nature | Range in air | Penetrating power | Ionising ability |
|---|---|---|---|---|
Alpha (α) | helium nucleus (2 protons, 2 neutrons) | a few cm | low; stopped by a thin sheet of paper | high |
Beta (β) | high-energy electron | a few m | moderate; stopped by a few mm of aluminium foil or Perspex | moderate |
Gamma (γ) | electromagnetic wave | infinite | high; reduced by a few cm of lead | low |
Penetrating power & shielding
Alpha, beta and gamma radiation have different properties
So they penetrate materials in different ways
This means they are each stopped by different materials
When materials are used to stop radiation, this is known as shielding
Shielding of alpha, beta and gamma radiation
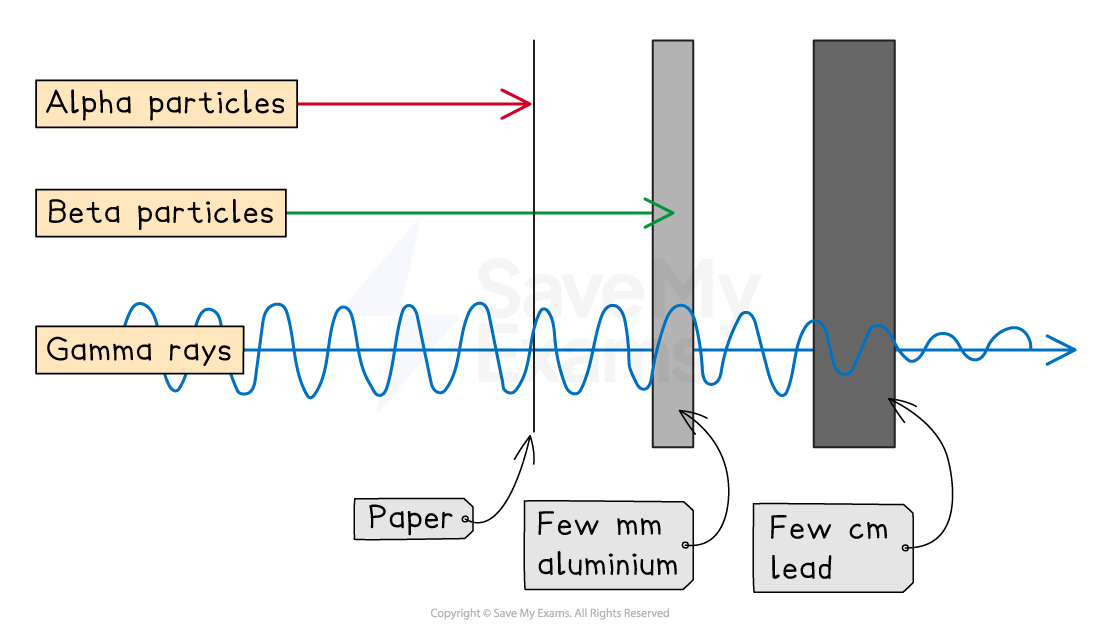
Alpha is stopped by paper, whereas beta and gamma pass through it
Beta is stopped by a few millimetres of aluminium
Gamma can pass through aluminium
Gamma rays are only partially stopped by thick lead
Examiner Tips and Tricks
It is important to note that beta particles are only stopped by aluminium if it is a few mm thick. They can pass though aluminium which is thinner than this. This concept often comes up in exam questions.
Worked Example
A student has an unknown radioactive source and is trying to determine which type of radiation it emits. Using a Geiger-Muller tube, they measure the count rate when the source is placed behind different materials.
Their results are shown in the table below:
| No material between source and detector | Paper between source and detector | 5 mm aluminium between source and detector | 5 mm lead between source and detector |
Count rate | 4320 | 4218 | 256 | 34 |
Which type of radiation is being given off by the source?
A Alpha particles
B Beta particles
C Gamma rays
D All of the above
Answer: B
Consider the diagram showing penetrating power from above
The answer is not A because the radiation passed through the paper almost unchanged
This means it is not alpha
The answer is not C or D because the aluminium decreased the count rate significantly
This means it is not gamma (gamma penetrates aluminium)
Therefore, the source must be beta particles
Examiner Tips and Tricks
Remembering the type of particle, penetration and ionising power for alpha, beta and gamma radiation is very important for your exam! Often the exam question will give some clues and you will have to choose which type of radiation it could be based on these.

Unlock more, it's free!
Was this revision note helpful?
