Controlling Variables in Chemistry (DP IB Chemistry): Revision Note
Controlling Variables in Chemistry
This is the practical application of the 'Controlled Variables' you identified in your design.
The main goal of controlling variables is to minimise systematic errors—errors that consistently shift your results in one direction.
By keeping all other factors constant, you can be:
More confident that any effect you measure is due to the change in your independent variable.
Ensure that the results are valid .
Ensure that you are conducting a fair test.
Methods of control
Appreciate when and how to calibrate measuring apparatus
To ensure your data is accurate, the instruments you use must be checked and calibrated.
Calibration is the process of checking an instrument's readings against a known, reliable standard and adjusting it if necessary.
You should consider calibration for key instruments in your design:
pH meters
Before taking readings, a pH meter must be calibrated using at least two standard buffer solutions (e.g., at pH 4.00 and 7.00).
This adjusts the probe's output to give correct pH values.
Digital thermometers and sensors
Their accuracy can be checked by placing them in boiling, distilled water (which should read 100.0°C at standard pressure) and a beaker of crushed ice (0.0°C).
A data logger connected to a temperature probe. 
A digital thermometer.
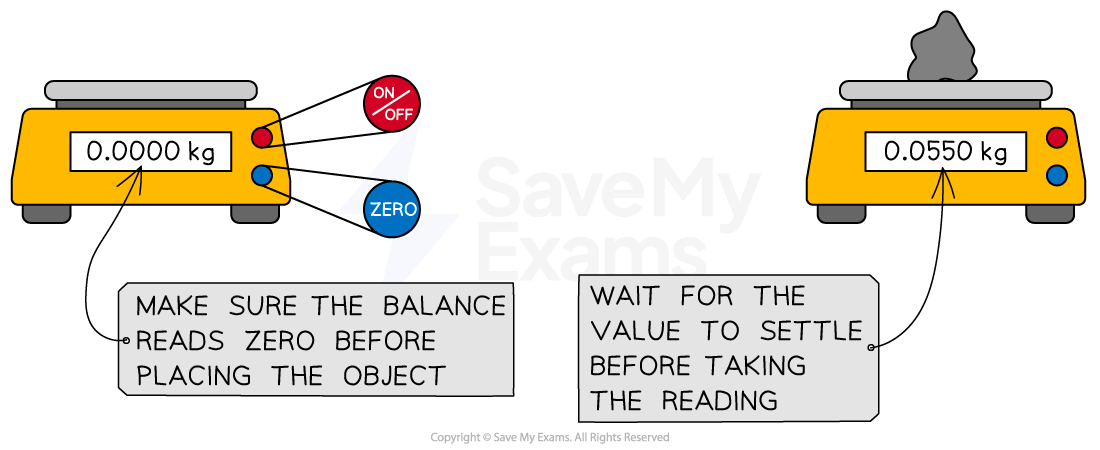
Digital balances
Must always be zeroed (tared) before use to ensure you are only measuring the mass of your chemical.
Appreciate when and how to maintain constant environmental conditions
The conditions in the laboratory can affect your results. Key environmental conditions to control include temperature, air pressure, and air currents (draughts).
Maintaining temperature
For many reactions, especially in kinetics, temperature is the most important variable to control as it directly affects the rate.
The best way to do this is to use a thermostatically controlled water bath.
Place all your reactant solutions in the water bath for a set amount of time (e.g., 5–10 minutes) before mixing them to ensure they are all at the same starting temperature.
Controlling air currents
Draughts from windows or air conditioning can cool a reaction vessel, causing significant errors in calorimetry experiments.
This can be controlled by closing windows or using a draught shield around the apparatus.
Sometimes, a variable cannot be perfectly controlled (e.g., ambient room temperature might fluctuate slightly).
In these cases, the best practice is to monitor and record it.
You can then discuss its potential impact in your evaluation.
Appreciate when and how to insulate against heat loss or gain
In any calorimetry experiment (e.g., measuring enthalpy of neutralisation or combustion), the biggest source of error is unwanted heat exchange with the surroundings.
Insulating the system is crucial for collecting accurate temperature data.
Common insulation techniques include:
Using a polystyrene cup as the calorimeter instead of a glass beaker, as polystyrene is a much better thermal insulator.
Placing the polystyrene cup inside a larger glass beaker to provide stability and an insulating layer of air.
Using a lid (e.g., made of cardboard or plastic) with holes for the thermometer and stirrer. This minimises heat loss by evaporation and convection.
Worked Example
Research question:
"How does the strength of a monoprotic acid affect the standard enthalpy of neutralisation?"
Below is how you would identify and control the key variables for this experiment to ensure a fair test:
Accuracy of temperature readings
Method of control:
The digital thermometer will be checked against boiling water before use.
Justification:
This is a form of calibration to ensure the instrument is accurate and that the measured ΔT is valid.
Initial temperature of reactants
Method of control:
The acid and base solutions will be placed in a 25°C water bath for 10 minutes before being mixed.
Justification:
This ensures that both reactants start at the same, controlled temperature for every trial.
Heat loss to the surroundings
Method of control:
The reaction will be performed in a polystyrene cup with a lid, which is placed inside a 250 cm3 glass beaker.
A draught shield will be placed around the apparatus.
Justification:
This combination of techniques insulates the system, minimising heat loss through conduction, convection, and evaporation, which is the largest source of systematic error.
Volume of reactants
Method of control:
A 25.00 cm3 volumetric pipette will be used to measure the acid and a 25.0 cm3 measuring cylinder for the base.
Justification:
Using precise volumetric apparatus for at least one reactant ensures that the reacting volumes are kept accurately constant for each trial.
Worked Example
Research question:
"What is the effect of hydrochloric acid concentration on the rate of reaction with a magnesium ribbon?"
Below is how you would identify and control the key variables for this experiment.
Temperature of the acid
Method of control:
All acid solutions will be placed in a 25°C water bath for 10 minutes before the magnesium is added.
Justification:
The rate of reaction is highly sensitive to temperature.
Maintaining a constant temperature ensures that any change in rate is solely due to concentration, not temperature fluctuations.
Surface area of the magnesium
Method of control:
Use 2.0 cm lengths of magnesium ribbon, cut from the same roll, for every trial.
Polish each strip lightly with sandpaper to remove any oxide layer.
Justification:
The surface area of a solid reactant affects the number of particles exposed for collision.
Using identical lengths from the same source and removing the oxide layer ensures the surface area is consistent.
Volume of the acid
Method of control:
Use a 50.0 cm3 measuring cylinder to add exactly 50.0 cm3 of HCl solution for each trial.
Justification:
The volume of the acid must be kept constant to ensure that the total number of acid particles is proportional to the concentration and is not an additional variable.
Examiner Tips and Tricks
Be specific about how you control a variable.
Simply stating "I will control the temperature" is not enough.
You must explain the method: "A water bath set to 25°C will be used to maintain a constant temperature."
Don't forget simple controls.
Important controls include:
Using the same piece of equipment (e.g., the same digital balance) for all measurements of a certain type
Having the same person read the measurement (e.g., timing an endpoint) to reduce subjective error.
Justify your controls.
For top marks, briefly explain why controlling a particular variable is important for the validity of your experiment.
For example, "Temperature was controlled because it affects the kinetic energy of particles and therefore the rate of reaction."
Prioritise your controls.
In your justification, focus your explanation on the variables that will have the most significant impact on your results (e.g., heat loss in calorimetry).
This demonstrates insight.

Unlock more, it's free!
Was this revision note helpful?
