Combustion of Alkanes (Cambridge (CIE) A Level Chemistry): Revision Note
Exam code: 9701
Combustion of Alkanes & the Environment
Cars’ exhaust fumes include toxic gases such as carbon monoxide (CO), oxides of nitrogen (NO/NO2) and volatile organic compounds (VOCs)
When released into the atmosphere, these pollutants have drastic environmental consequences damaging nature and health
Carbon monoxide
When oxygen supply is limited, carbon monoxide (CO) forms instead of carbon dioxide:
fuel + oxygen → carbon monoxide + water
For example, Incomplete combustion of propane:
propane + oxygen → carbon monoxide + water
C3H8 (l) + 3½O2 (g) → 3CO (g) + 4H2O (l)
Carbon monoxide is extremely dangerous because it is:
Colourless and odourless (can’t be seen or smelled)
Hard to detect without a sensor
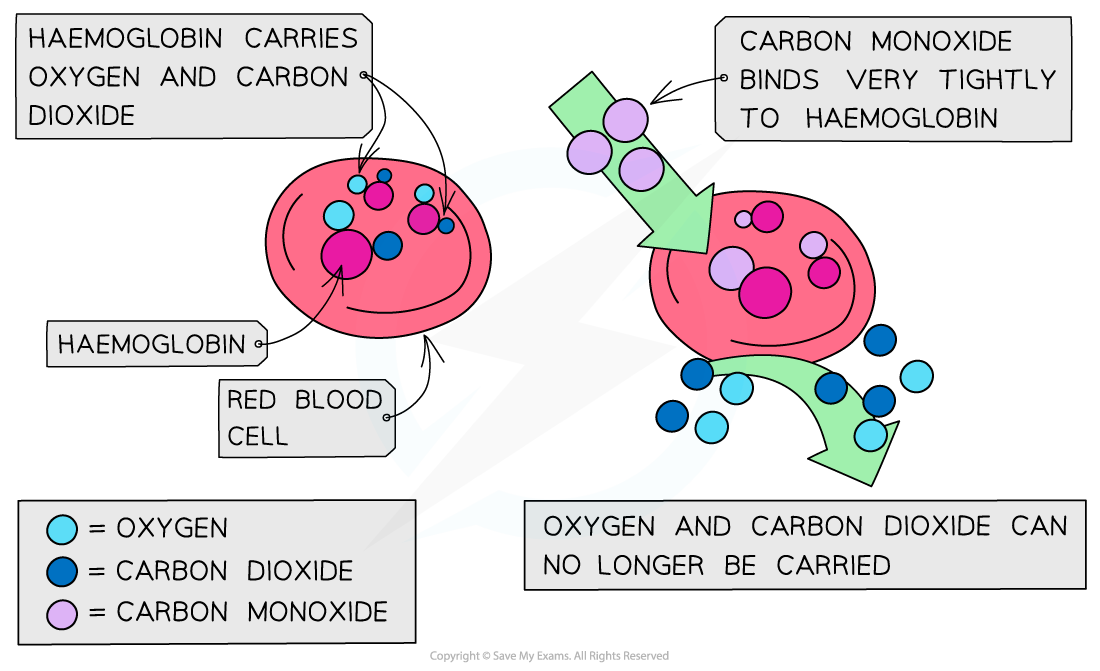
It is a toxic and poisonous gas that binds irreversibly to haemoglobin in the blood.
This prevents haemoglobin from carrying oxygen.
Lack of oxygen transport leads to:
Dizziness
Loss of consciousness
Potentially death if not treated
The effect of carbon monoxide on haemoglobin
Oxides of nitrogen
Normally, nitrogen is too unreactive to react with oxygen in air
In a car’s engine, high temperatures and pressures are reached, causing the oxidation of nitrogen to take place:
N2 (g) + O2 (g) → 2NO (g)
N2 (g) + 2O2 (g) → 2NO2 (g)
The oxides of nitrogen are then released in the car’s exhaust fumes into the atmosphere
Car exhaust fumes also contain unburnt hydrocarbons from fuels and their oxides (VOCs)
In the air, the nitrogen oxides can react with these VOCs to form peroxyacetyl nitrate (PAN) which is the main pollutant found in photochemical smog
PAN is also harmful to the lungs, eyes and plant life
Nitrogen oxides can also dissolve and react in water with oxygen to form nitric acid which is a cause of acid rain
Acid rain can cause corrosion of buildings, endanger plant and aquatic life (as lakes and rivers become too acidic) and directly damage human health
Catalytic removal
To reduce the amount of pollutants released in cars’ exhaust fumes, many cars are now fitted with catalytic converters
Precious metals (such as platinum) are coated on a honeycomb to provide a large surface area
The reactions that take place in the catalytic converter include:
Oxidation of CO to CO2:
2CO + O2 → 2CO2
or
2CO + 2NO → 2CO2 + N2
Reduction of NO/NO2 to N2:
2CO + 2NO → 2CO2 + N2
Oxidation of unburnt hydrocarbons:
CnH2n+2 + (3n+1)[O] → nCO2 + (n+1)H2O
Pollutants, their effect & removal summary
Carbon Monoxide (CO)
Formation:
Incomplete combustion of alkanes in car engines
Environmental consequence:
Toxic gas
Catalytic removal:
Oxidised to carbon dioxide (CO2):
2CO + O2 → 2CO2
2CO + 2NO → 2CO2 + N2
Oxides of Nitrogen (NO, NO2 etc.)
Formation:
Oxidation of nitrogen in car engines (due to high temperatures)
Environmental consequence:
Dissolve in water and react with oxygen to form acid rain
Catalytic removal:
Reduced to nitrogen gas:
2CO + 2NO → 2CO2 + N2
Volatile Organic Compounds (VOCs)
Formation:
Unburnt hydrocarbons from fuels
Oxides of these hydrocarbons formed in car engines
Environmental consequence:
React with oxides of nitrogen in the atmosphere to form PAN
Catalytic removal:
Oxidised to CO2 and H2O
General formula reaction:
CnH2n+2 + (3n + 1)[O] → nCO2 + (n + 1)H₂O
PAN (peroxyacyl nitrate)
Formation:
From the photochemical reaction of VOCs and nitrogen oxides in the atmosphere
Environmental consequence:
Contributes to photochemical smog
Catalytic removal:
Oxidise unburnt hydrocarbons
Reduce nitrogen oxides to prevent PAN formation
Examiner Tips and Tricks
Although CO2 is not a toxic gas, it is still a pollutant causing global warming and climate change

Unlock more, it's free!
Was this revision note helpful?
