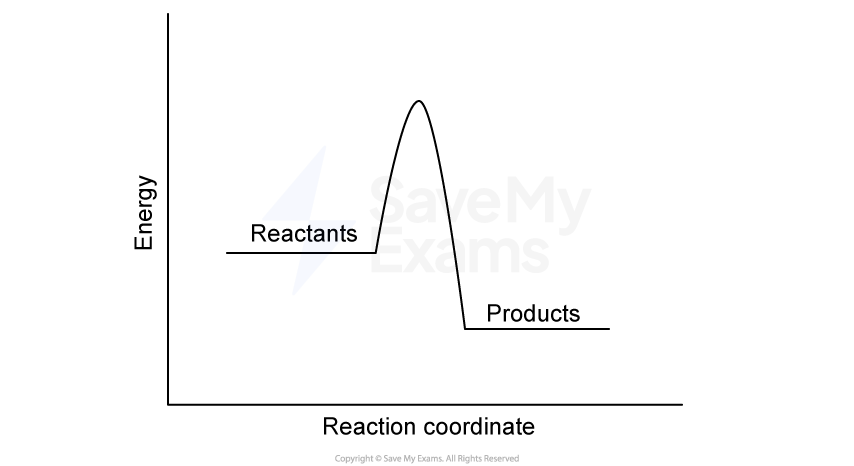
A car engine coolant system is designed to absorb excess heat from the engine and release it into the surrounding air. A 4.00 kg sample of coolant absorbs 125 kJ of heat, causing its temperature to rise from 25.0°C to 75.0°C.
Write the equation used to calculate heat transfer.
Determine the specific heat capacity of the coolant.
Explain why different substances have different specific heat capacities.
If the engine were cooled with water instead, would the temperature change be greater or smaller? Justify your answer.
Was this exam question helpful?