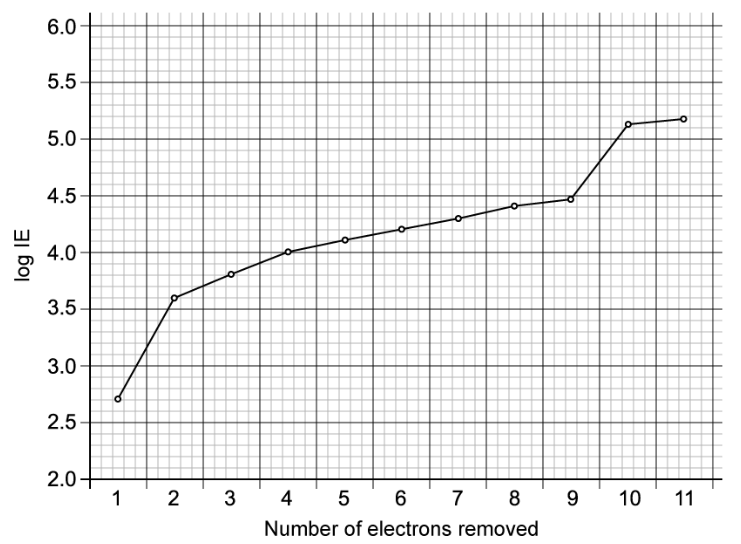
Element A has the following first six ionisation energies in kJ mol-1.
577, 1820, 2740, 11 600, 14 800, 18 400
i) Explain how you know that element A is in group 3 of the periodic table.
[1]
ii) Two elements B and C are in the same period as A, but B is in the group before A and C is in the group after A in the periodic table.
Give approximate first ionisation energies for elements B and C.
[2]
iii) Explain, using ideas of electronic structure, the difference in ionisation energy values of element A compared to elements B and C.
[2]