Preparation of Cyclohexene (OCR A Level Chemistry A): Revision Note
Exam code: H432
PAG 5.2: Preparation of cyclohexene
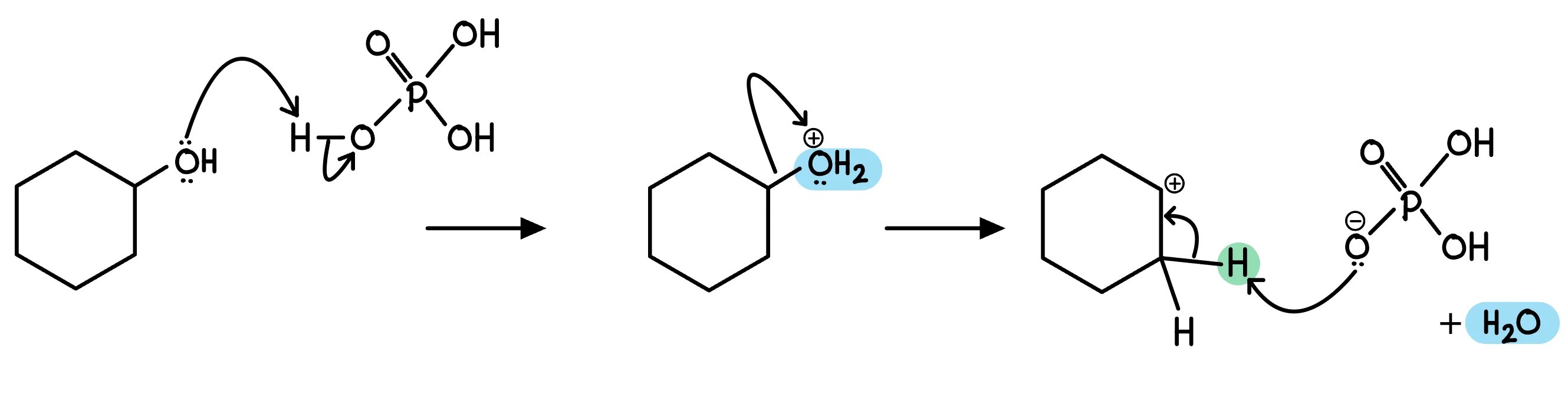
In this experiment, cyclohexene is prepared by dehydratng of cyclohexanol using an acid catalyst such as phosphoric acid
C6H11OH C6H10 + H2O
This is one of the most common methods of preparing alkenes
The crude product is contaminated with:
Water
Unreacted alcohol
Phosphoric acid
Some side products
Wash the crude product with saturated sodium hydrogen carbonate to remove acid
Then, wash with water to remove any remaining carbonate
Add solid calcium chloride to remove residual water
The elimination mechanism is shown below
You do not need to learn it, but you may be asked to draw in charges or lone pairs
Preparation of cyclohexene
Measure approximately 20 cm3 of cyclohexanol into a weighed 50 cm3 pear-shaped flask
Use a pipette to slowly add 8 cm3 of concentrated phosphoric acid
Add a few anti-bumping granules to the flask
Assemble the distillation apparatus so you can distil the contents of the flask
Heat the flask gently using an electric heater or water bath
Collect the distillate

Purification of cyclohexene
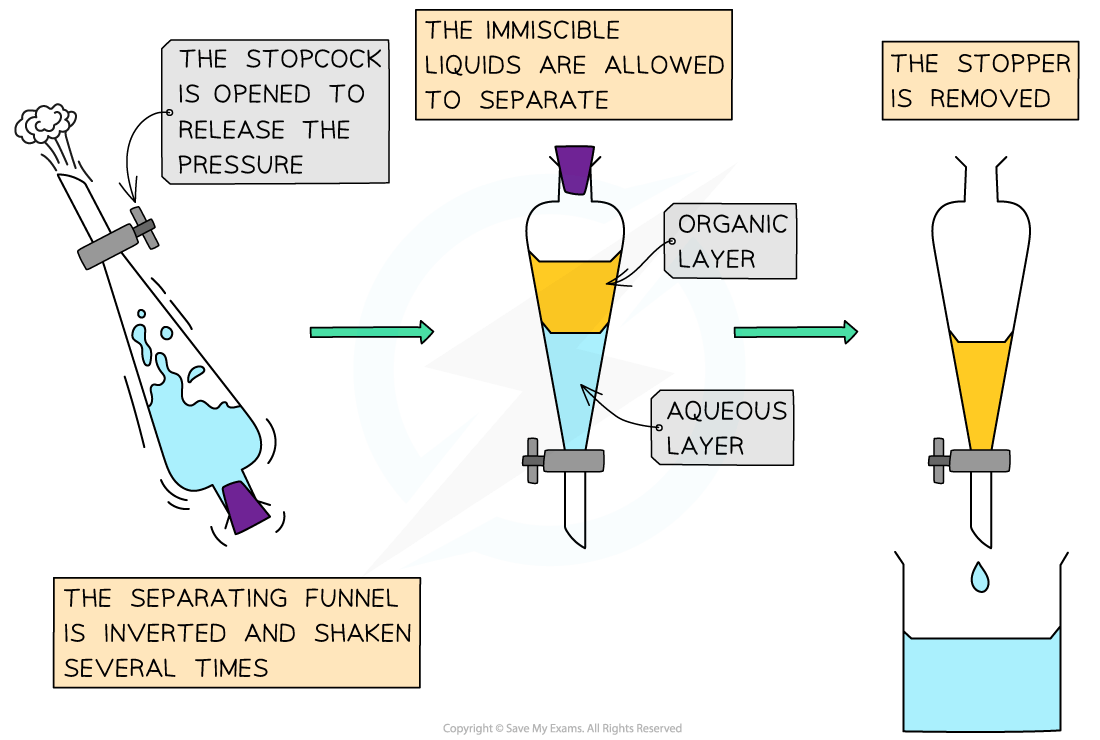
Pour the distillate into a separating funnel
Add 10 cm3 of sodium hydrogen carbonate solution
This is to remove unreacted acid and acidic impurities
Shake the funnel and allow the layers to separate, releasing pressure occasionally
Carefully run off the lower aqueous layer
Transfer the upper organic layer (crude cyclohexene) into a conical flask
Add solid anhydrous calcium chloride to remove water
Stopper the flask, shake it gently, and allow it to stand
Once the liquid is clear, transfer it to a clean, dry beaker
Test a small portion of the distillate with bromine water to confirm the presence of an alkene
A positive result is a colour change from orange-brown to colourless
Practical skills reminder
This practical demonstrates the purification of a liquid product using:
A separating funnel to remove aqueous impurities
Washing steps with sodium hydrogen carbonate and water
A drying agent (anhydrous calcium chloride) to remove water
Final distillation to isolate and test the purified alkene

Unlock more, it's free!
Was this revision note helpful?
