Oxidation of Ethanol (OCR A Level Chemistry A): Revision Note
Exam code: H432
PAG 5.3: Oxidation of ethanol
Primary alcohols can be oxidised to form aldehydes, which can undergo further oxidation to form carboxylic acids
For example:
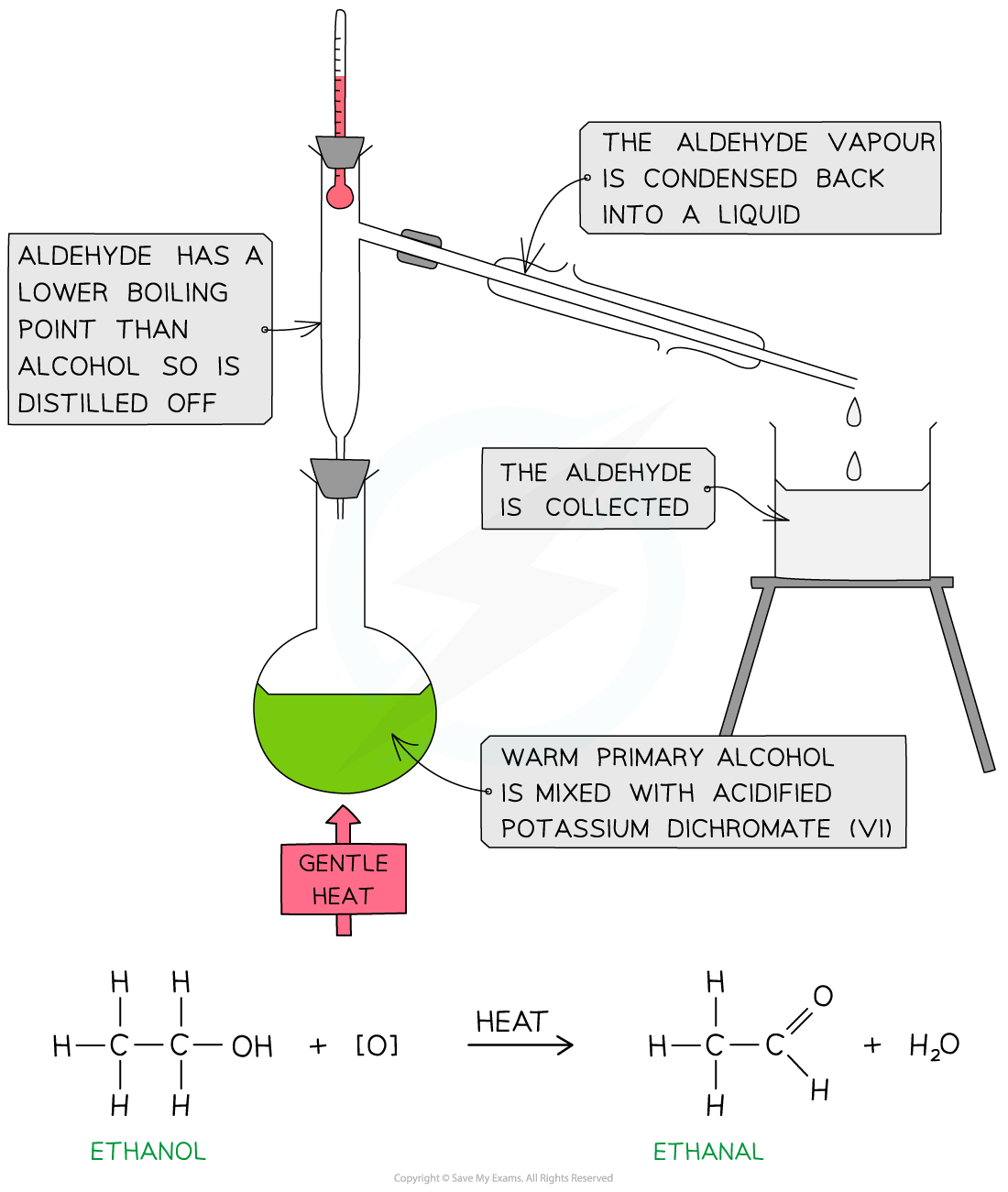
Oxidising ethanol produces ethanal
Ethanal can then be oxidised further to ethanoic acid
Synthesis and purification of ethanal and ethanoic acid
Add 20 cm3 of acidified potassium dichromate(VI) solution (K2Cr2O7) to a 50 cm3 pear-shaped flask
Cool the flask in an ice bath
Set up the reflux apparatus while keeping the flask cool
Add anti-bumping granules to the flask
Measure 1 cm3 of ethanol
Using a pipette, add the ethanol dropwise to the reflux condenser
Once all the ethanol has been added, remove the ice bath and allow the flask to warm to room temperature
Position the flask over an electric heater or in a water bath
Heat for 20 minutes
Ethanol is flammable - avoid naked flames
Use an electric heater or water bath for safety
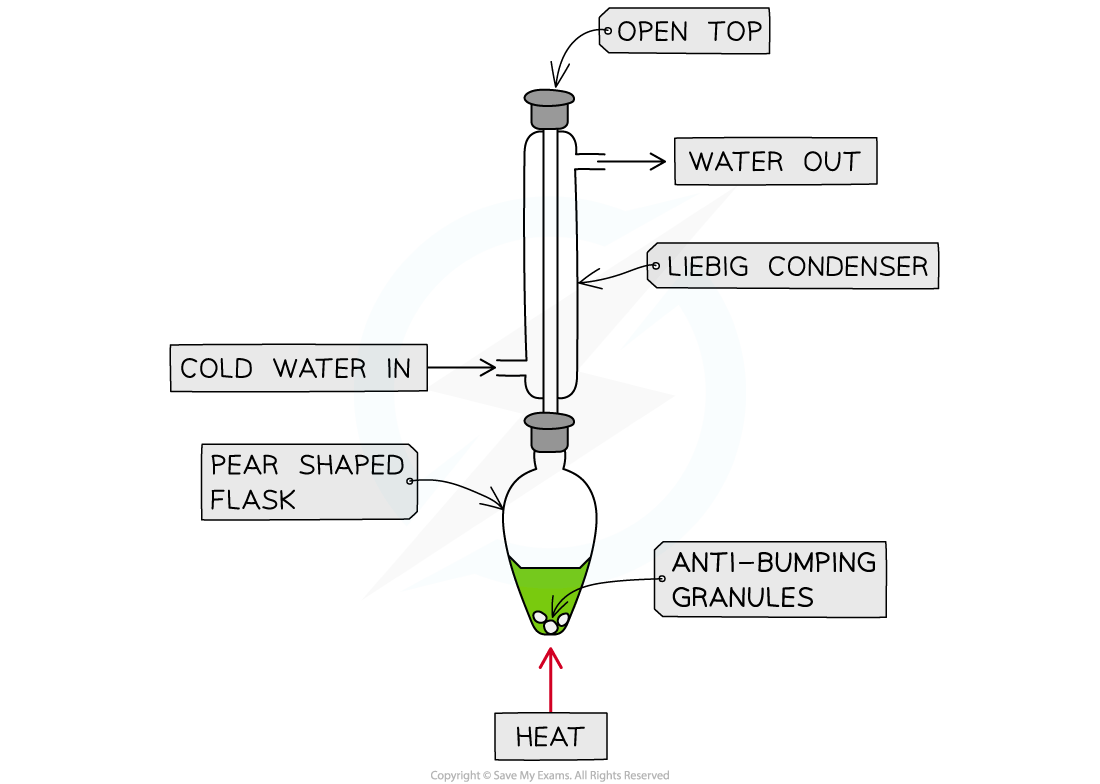
Purify the product using distillation apparatus:
Practical skills reminder
This practical demonstrates purification of a liquid product using:
Controlled reflux to ensure complete oxidation of the alcohol
Distillation to isolate and collect the target liquid (ethanal or ethanoic acid)
Appropriate safety measures for heating flammable reagents

Unlock more, it's free!
Was this revision note helpful?
