Electronegativity & Bond Polarity (OCR A Level Chemistry A): Revision Note
Exam code: H432
Electronegativity trends
Electronegativity is the power of an atom to attract the pair of electrons in a covalent bond towards itself
The electron distribution in a covalent bond between elements with different electronegativities will be unsymmetrical
This phenomenon arises from the ability of the positive nucleus to attract the negatively charged electrons, in the outer shells, towards itself
The Pauling scale is used to assign a value of electronegativity for each atom
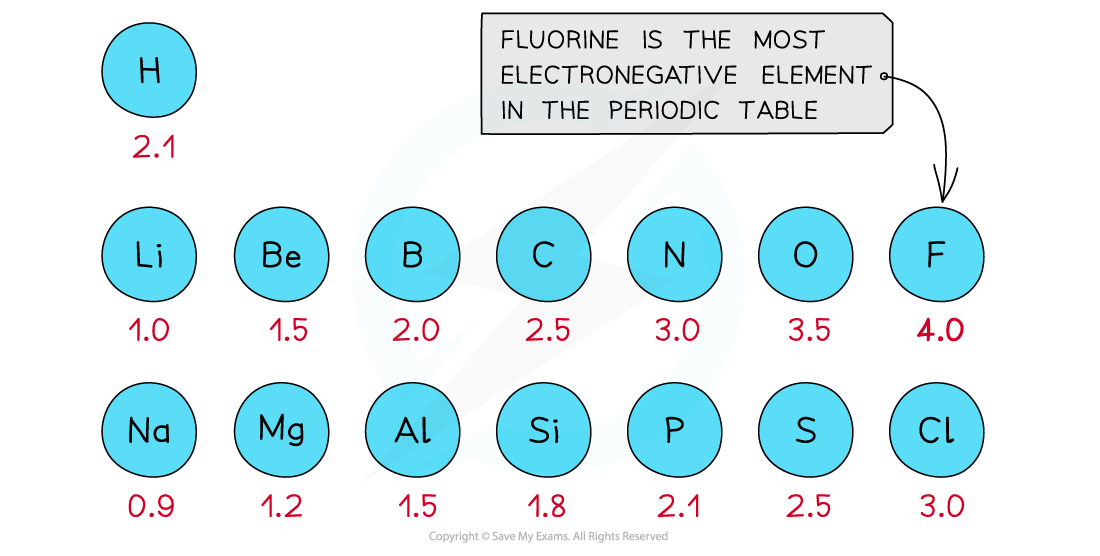
Fluorine is the most electronegative atom on the Periodic Table, with a value of 4.0 on the Pauling Scale
It is best at attracting electrons towards itself when covalently bonded to another atom
There are various factors which will affect the electronegativity of an element
Nuclear charge
Attraction exists between the positively charged protons in the nucleus and negatively charged electrons found in the energy levels of an atom
An increase in the number of protons leads to an increase in nuclear attraction for the electrons in the outer shells
Therefore, an increased nuclear charge results in an increased electronegativity
Atomic radius
The atomic radius is the distance between the nucleus and electrons in the outermost shell
Electrons closer to the nucleus are more strongly attracted towards its positive nucleus
Those electrons further away from the nucleus are less strongly attracted towards the nucleus
Therefore, an increased atomic radius results in a decreased electronegativity
Shielding
Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus
Sodium (period 3, group 1) has higher electronegativity than caesium (period 6, group 1) as it has fewer shells and therefore the outer electrons experience less shielding than in caesium
Thus, an increased number of inner shells and subshells will result in a decreased electronegativity
Electronegativity varies across periods and down the groups of the periodic table
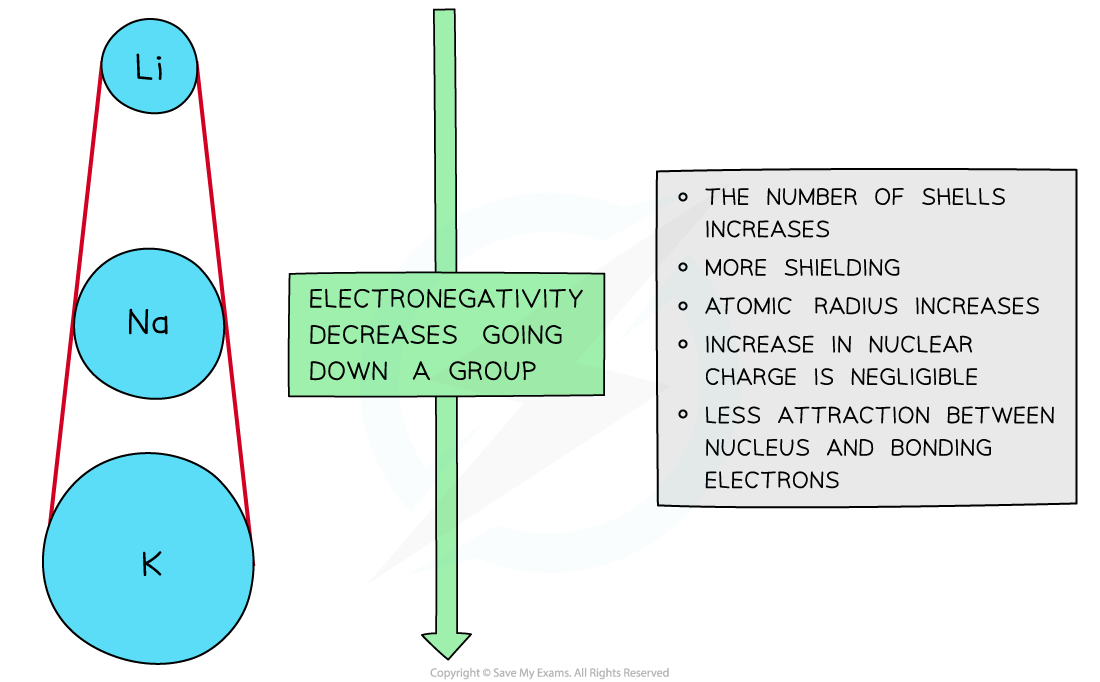
Down a group
There is a decrease in electronegativity going down the group
The nuclear charge increases as more protons are being added to the nucleus
However, each element has an extra filled electron shell, which increases shielding
The addition of the extra shells increases the distance between the nucleus and the outer electrons resulting in larger atomic radii
Overall, there is decrease in attraction between the nucleus and outer bonding electrons
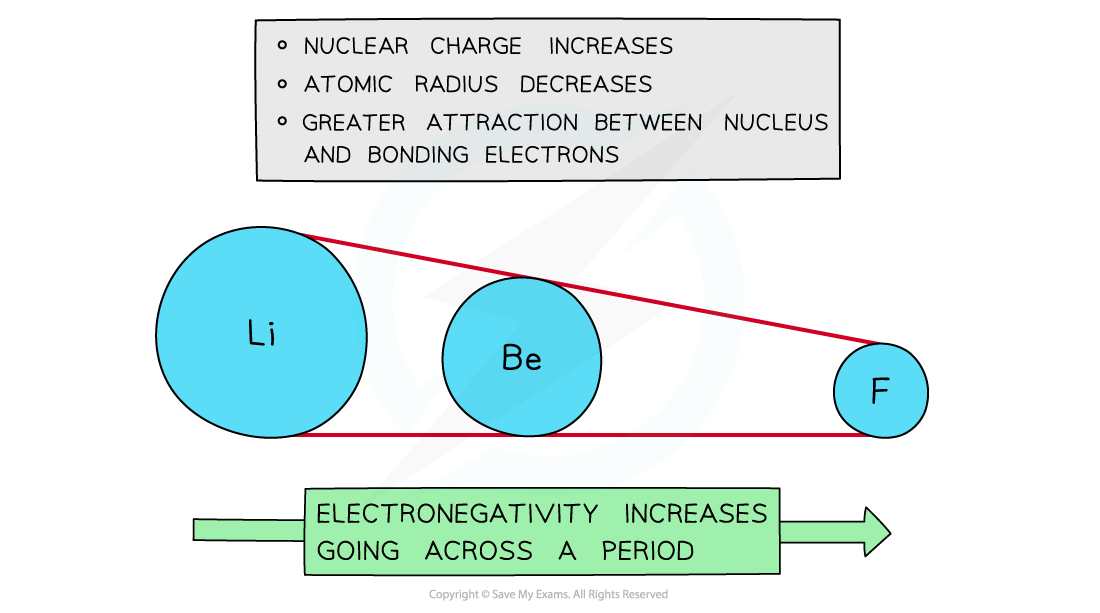
Across a period
Electronegativity increases across a period
The nuclear charge increases with the addition of protons to the nucleus
Shielding remains relatively constant across the period as no new shells are being added to the atoms
The nucleus has an increasingly strong attraction for the bonding pair of electrons of atoms across the period of the periodic table
This results in smaller atomic radii
Bond polarity
Polarity
When two atoms in a covalent bond have the same electronegativity the covalent bond is nonpolar
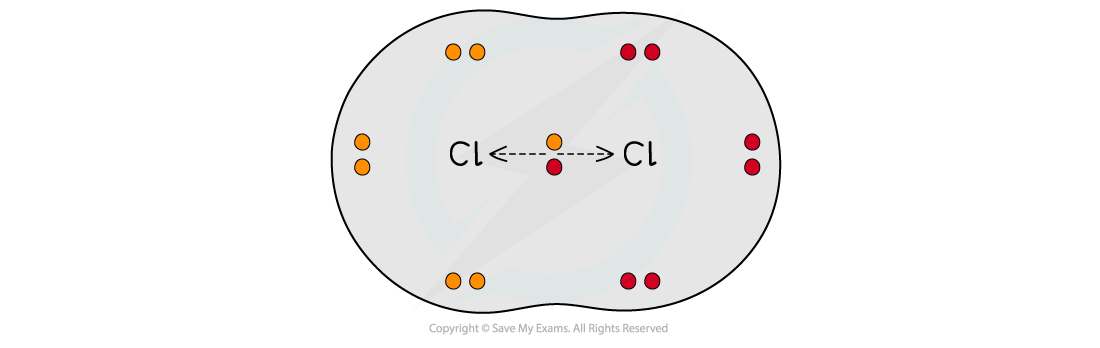
When two atoms in a covalent bond have different electronegativities the covalent bond is polar and the electrons will be drawn towards the more electronegative atom
As a result of this:
The negative charge centre and positive charge centre do not coincide with each other
This means that the electron distribution is asymmetric
The less electronegative atom gets a partial charge of δ+ (delta positive)
The more electronegative atom gets a partial charge of δ- (delta negative)
The greater the difference in electronegativity the more polar the bond becomes
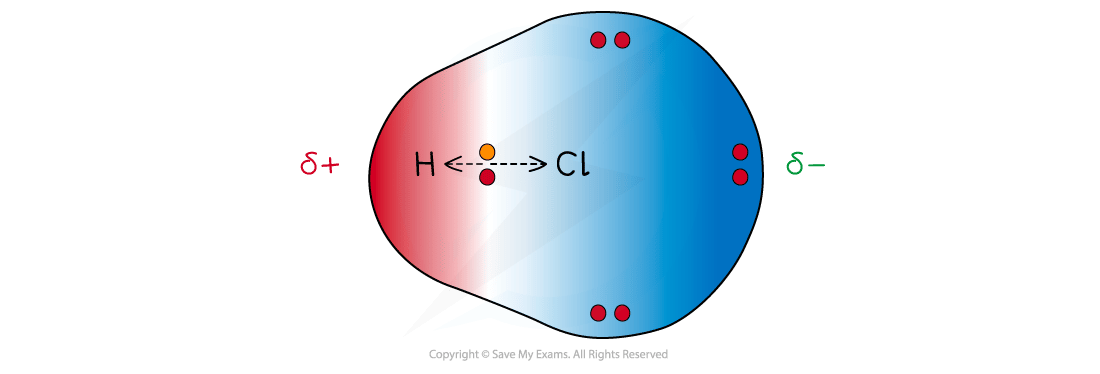
Dipole moment
The dipole moment is a measure of bond polarity
Dipole moments can be shown using the following sign:

The sign shows:
The direction of the dipole moment
The arrow points to the δ- (delta negative) end of the dipole
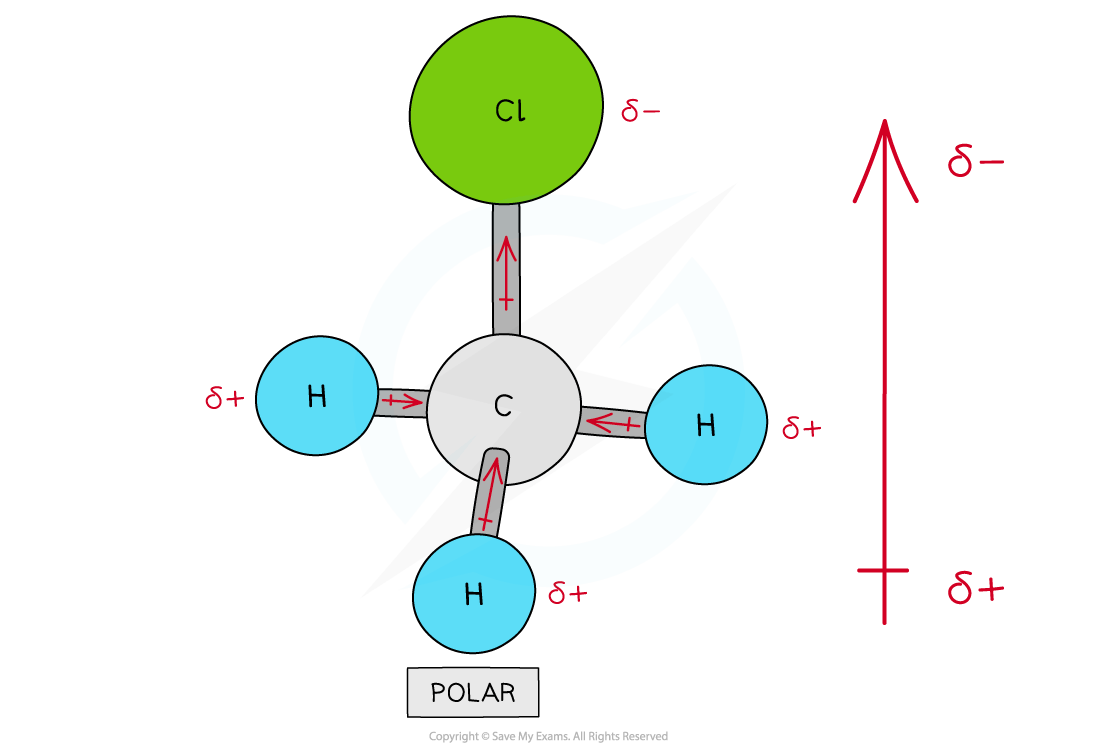
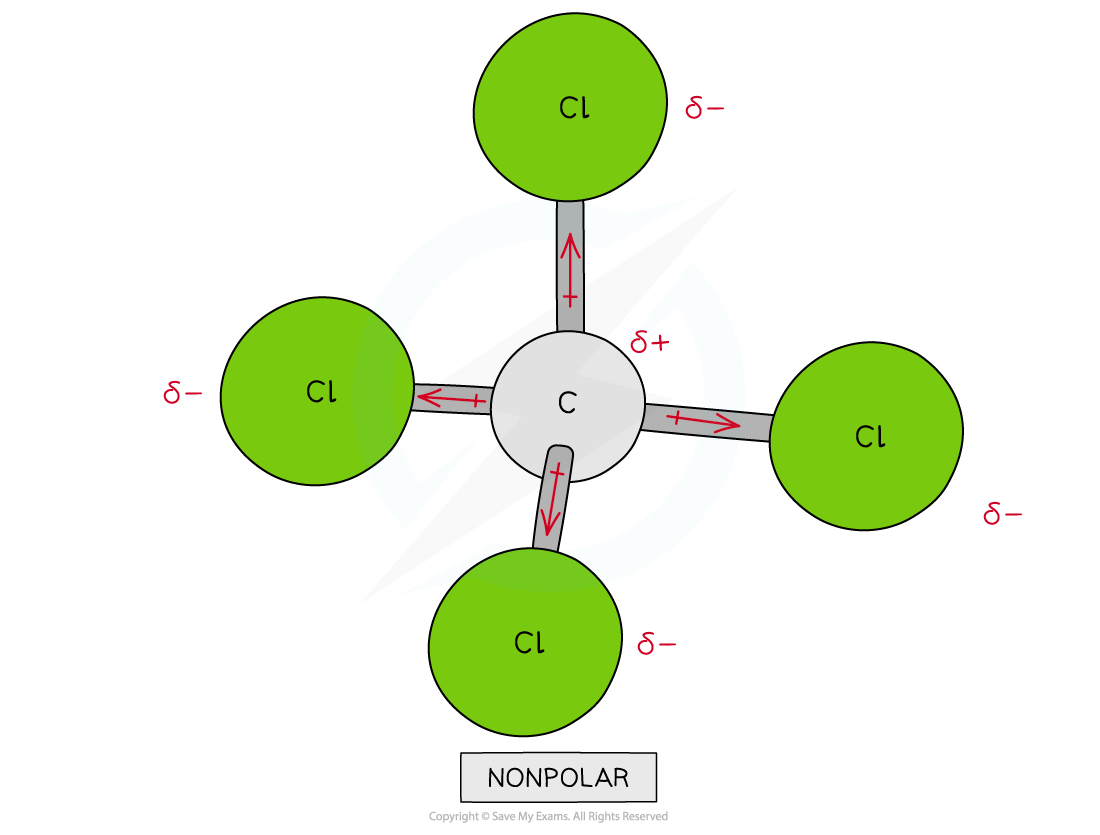
Assigning polarity to molecules
To determine whether a molecule with more than two different atoms is polar, the following things have to be taken into consideration:
The polarity of each bond
How the bonds are arranged in the molecule
Some molecules have polar bonds but are overall not polar because the polar bonds in the molecule are arranged in such way that the individual dipole moments cancel each other out
Bond polarity and molecular dipole summary
Type of Bond | Electronegativity Difference | Polarity |
|---|---|---|
Cl–Cl | 0 | Non-polar |
H–Cl | Moderate | Polar bond |
CH3Cl | Moderate | Polar molecule |
CCl4 | Moderate | Non-polar molecule |

Unlock more, it's free!
Was this revision note helpful?
