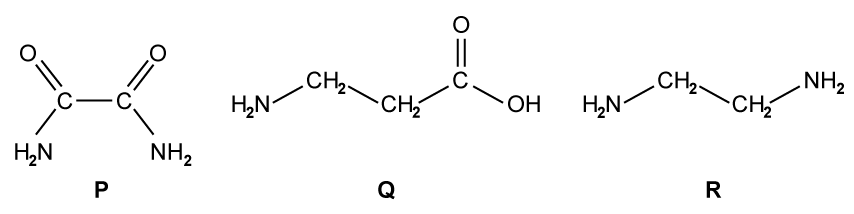
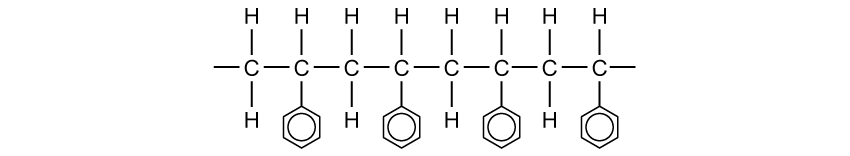
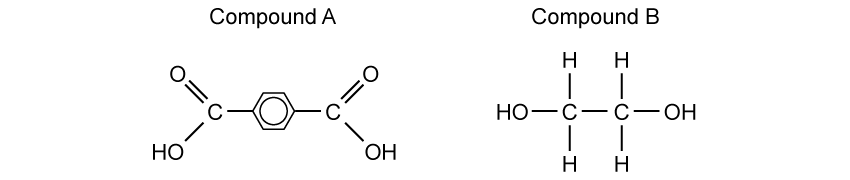
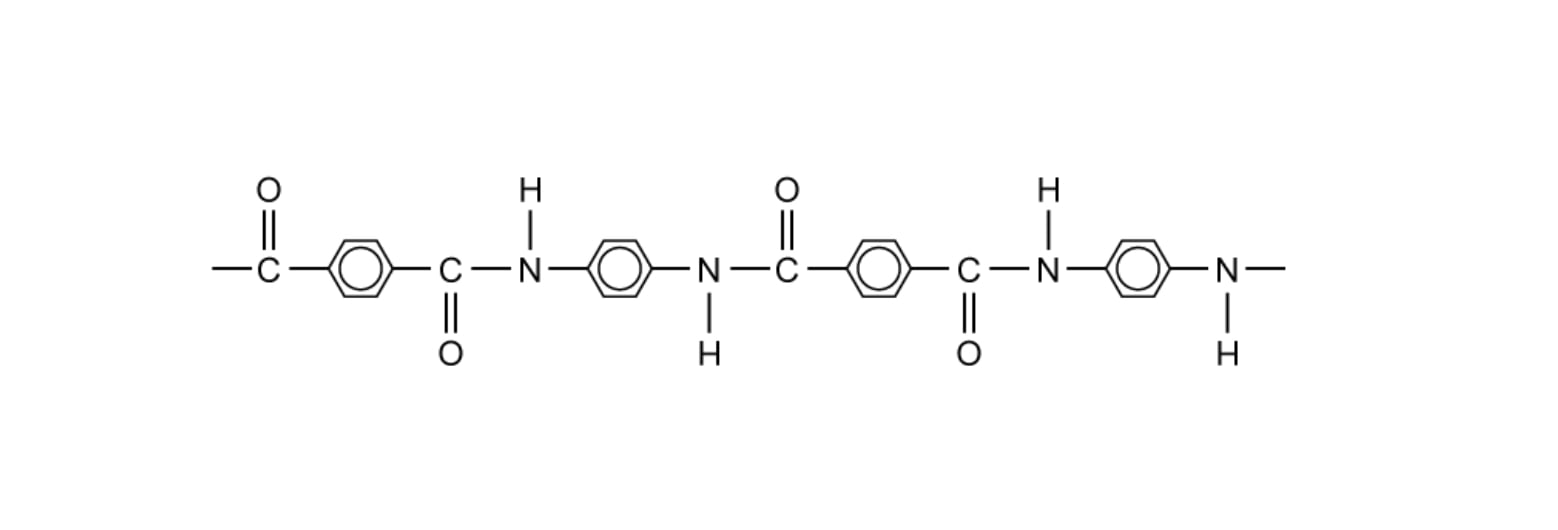
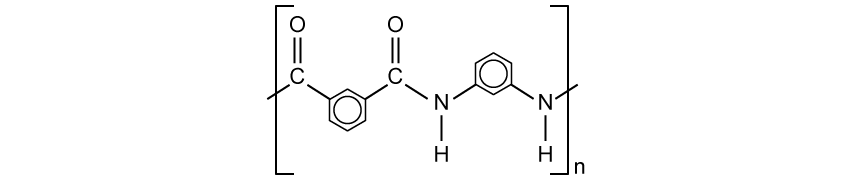
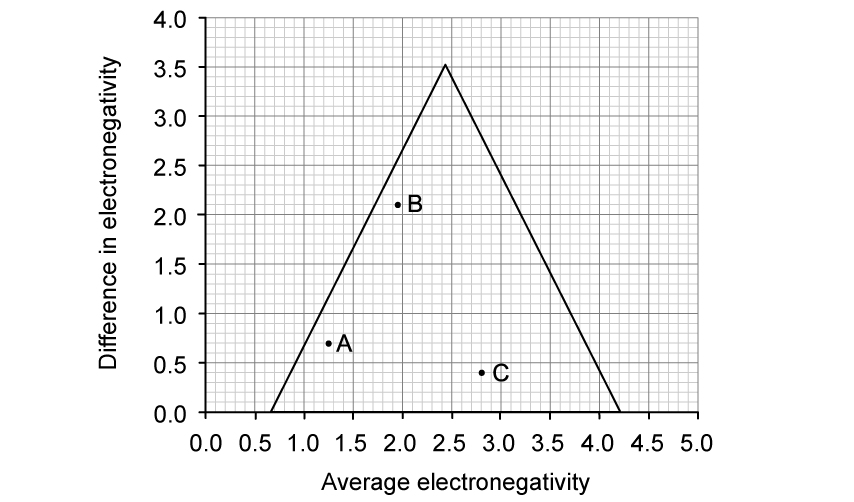
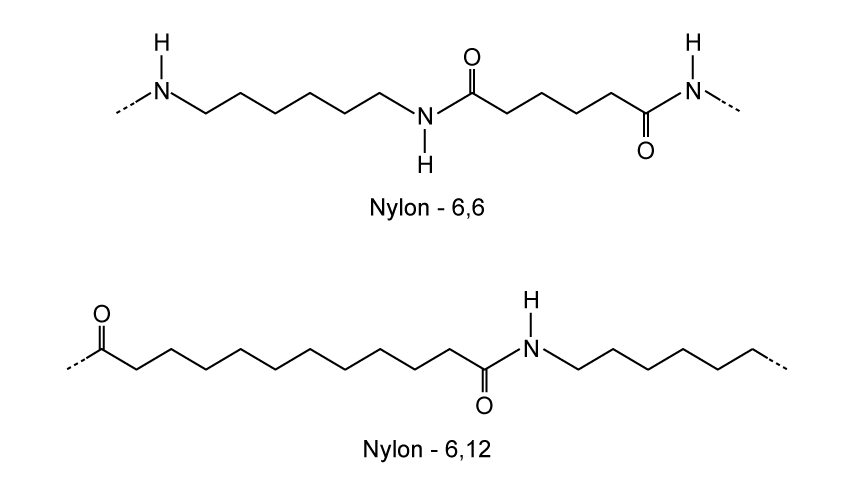
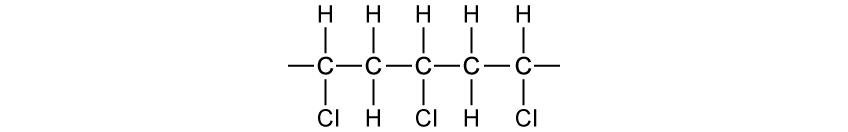
State why elements are found at the bottom of a triangular bonding diagram.
Suggest the position of ionic and covalent materials within a triangular bonding diagram. Explain your answer.
Name the x-axis and y-axis on a standard triangular bonding diagram.
x-axis: ..................................................
y-axis: ..................................................
Using sections 9 and 17 of the data booklet, plot nitrogen trifluoride (NF3) on the triangular bonding diagram.
Was this exam question helpful?