Hess's Law (DP IB Chemistry): Revision Note
Hess's Law
In 1840, the Russian chemist Germain Hess formulated a law which went on to be known as Hess’s Law
This went on to form the basis of one of the laws of thermodynamics. The first law of thermodynamics relates to the Law of Conservation of Energy
It is sometimes expressed in the following form:
Energy cannot be created or destroyed, it can only change form
This means that in a closed system, the total amount of energy present is always constant
Hess’s law can be used to calculate the standard enthalpy change of a reaction from known standard enthalpy changes
Hess’s Law states that:
"The total enthalpy change in a chemical reaction is independent of the route by which the chemical reaction takes place as long as the initial and final conditions are the same."
This means that whether the reaction takes place in one or two steps, the total enthalpy change of the reaction will still be the same
Diagram to show Hess's Law
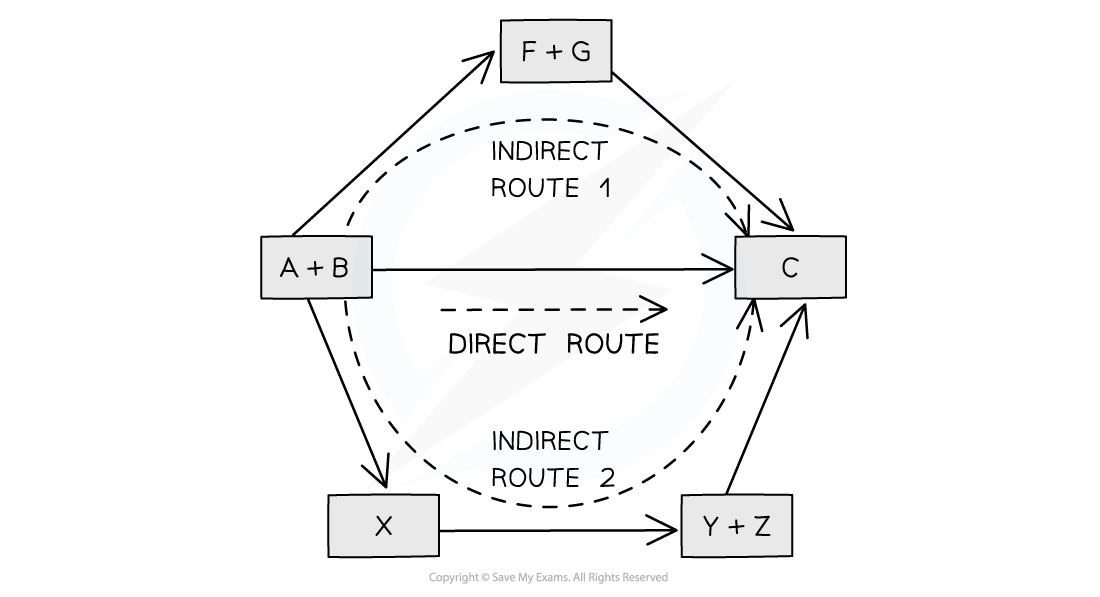
Hess’ Law is used to calculate enthalpy changes which can’t be found experimentally using calorimetry, eg:
3C (s) + 4H2 (g) → C3H8(g)
ΔHf (propane) can’t be found experimentally as hydrogen and carbon don’t react under standard conditions
Calculating ΔHr from ΔHf using Hess’s Law energy cycles
Diagram to show Hess's Law

According to Hess’s Law, the total enthalpy change is the same regardless of the pathway taken, because energy is conserved
There are two possible routes to form the products from the elements:
1. Direct formation (one-step route):
Elements → Products
Enthalpy change: ΔH2
2. Indirect formation (two-step route via reactants):
Elements → Reactants → Products
Enthalpy change: ΔH1 + ΔHr
By Hess’s Law:
ΔH2 = ΔH1 + ΔHr
Rearranged to solve for the enthalpy change of reaction (ΔHᵣ):
ΔHr = ΔH2−ΔH1
Examiner Tips and Tricks
You do not need to learn Hess's Law word for word as it is not a syllabus requirement, but you do need to understand the principle as it provides the foundation for all the problem solving in Chemical Energetics

Unlock more, it's free!
Was this revision note helpful?
