Time of Flight Mass Spectrometry (AQA AS Chemistry): Revision Note
Exam code: 7404
Time of Flight (TOF) Mass Spectrometry
Mass spectrometry is a powerful analytical technique
It is the most useful instrument for the accurate determination of:
The relative atomic mass (Ar) of an element
The relative molecular mass (Mr) of a molecule
As a sample passes through the mass spectrometer, a spectrum is produced
The spectrum plots the abundance of ions against their mass-to-charge ratio (m/z)
The peak with the highest m/z value is often the molecular ion peak (M+)
The tallest peak in the spectrum is called the base peak
Principles of TOF mass spectrometry
The entire apparatus is kept under a high vacuum to prevent ions from colliding with air molecules.
There are 4 key stages:
Ionisation
Acceleration
Ion drift
Detection
Ionisation
The sample is converted into positive ions.
The two main methods are electron impact and electrospray ionisation
Electron impact:
This is used for elements and low-mass compounds
High-energy electrons bombard the vaporised sample from an electron gun
The high-energy electrons knock an electron off each particle to form a 1+ ion:
X (g) → X+ (g) + e-
The 1+ ions formed are called molecular ions (M+)
This high-energy process can also cause the M+ ion to break into smaller pieces, known as fragments
Fragment ions also pass through a TOF mass spectrometer and appear on the final mass spectrum
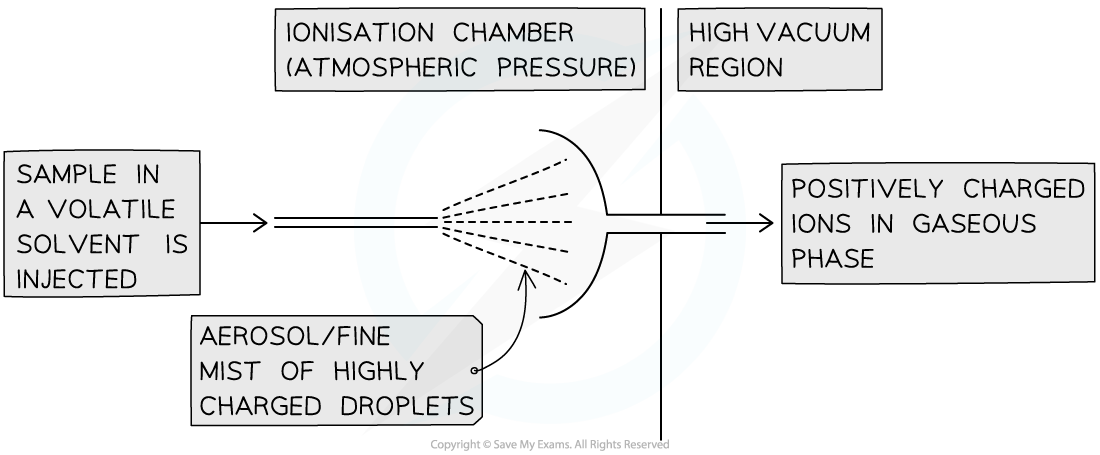
Electrospray ionisation (ESI):
This is used for higher-mass compounds (like proteins) to prevent fragmentation
This is a 'soft ionisation' technique
The sample is dissolved in a volatile solvent and injected through a high-voltage needle
This causes the particles to gain a proton (H+) from the solvent:
M (g) + H+ → MH+ (g)
Examiner Tips and Tricks
In electrospray ionisation, the mass of the detected ion will be the relative molecular mass of the sample + 1 (Mr + 1)
Acceleration
The positive ions are:
Attracted towards a negatively charged plate
Accelerated by an electric field
The key principle is that all ions are accelerated to have the same kinetic energy (KE)
Since KE = ½mv2:
Ions with a lower mass have a higher velocity
Ions with a higher mass have a lower velocity
Ion drift
The accelerated ions pass through a hole in the negatively charged plate and travel down a flight tube
The flight tube is a region with no electric field
The time it takes for an ion to travel this distance is its time of flight
Ions with a lower mass-to-charge ratio (m/z):
Travel faster
Have a shorter time of flight

Detection
The ions arrive at a detector (an electron multiplier)
When an ion hits the detector, it gains an electron, which generates a small electric current
The size of this current is directly proportional to the abundance of that specific ion
A computer records the time of flight and relative abundance for each ion to produce the mass spectrum
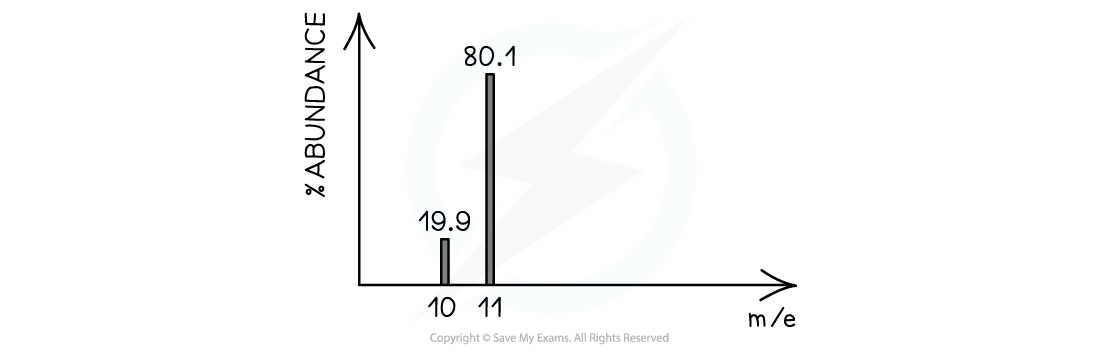
Example mass spectrum of boron
Key calculations for TOF mass spectrometry
TOF mass spectrometry uses two key equations:
Kinetic energy:
KE = ½mv2
Where:
KE = kinetic energy of the particles (J)
m = mass of the particles (kg)
v = velocity of the particles (ms-1)
Velocity:
v =
Where:
v = velocity of the particles (ms-1)
d = the length of the flight tube (m)
t = time of flight of the particles (s)
The kinetic energy and velocity equations are often combined
KE = ½mv2 rearranges to:
v =
v =
rearranges to:
t =
Substituting v into the t =
equation gives:
t = d
Unit conversions are crucial:
The mass (m) of the ion must be in kilograms (kg)
The length of the flight tube (d) must be in metres (m)
Calculating the mass of a single ion:
To convert the relative isotopic mass to the correct mass in kg:
Divide the relative isotopic mass by the Avogadro constant (L) (6.022 x 1023)
Divide the result by 1000 to convert it from grams to kilograms
Worked Example
In a TOF mass spectrometer, the time of flight, t, of an ion is shown by the
equation
In this equation d is the length of the flight tube, m is the mass, in kg, of an ion, and E is the kinetic energy of the ions.
In this spectrometer, the kinetic energy of an ion in the flight tube is 1.013 × 10−13 J
The time of flight of a 49Ti+ ion is 9.816 × 10−7 s
Calculate the time of flight of the 47Ti+ ion.
Give your answer to the appropriate number of significant figures.
Answer
Rearrange the given expression,
Substitute the known values,
Simplify,
Find the TOF for 47Ti+,
Examiner Tips and Tricks
Remember: All particles are accelerated to the same kinetic energy
The time of flight is proportional to the square root of the mass of the ions
Lighter ions travel faster and have a shorter time of flight
Heavier ions travel more slowly and have a longer time of flight

Unlock more, it's free!
Was this revision note helpful?
