Applications of Hess’s Law (AQA AS Chemistry): Revision Note
Exam code: 7404
Did this video help you?
Hess's Law Calculations
You must make sure that you can apply Hess' Law effectively and calculate enthalpy changes in different situations
Remember - it is the data that is important
Check whether the data you have been given is formation data or combustion data, and then complete the cycle or calculation according to that
Calculating ΔHf from ΔHc using Hess’s Law energy cycles
It can be difficult to find the enthalpy change of formation of compounds experimentally
However, many enthalpy changes of combustion can be measured experimentally so these can be used to find the enthalpy of formation
To do this, we follow these steps:
Write the equation for the formation of the compound
Write the combustion products below the equation
Draw downward pointing arrows from each substance to its combustion products
Write values on the arrows and multiply by the number of moles
In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction
Worked Example
Using the data provided, calculate the standard enthalpy change of formation, ΔHf, of propanone.
3C (s) + 3H2 (g) + ½ O2 (g) → CH3COCH3 (l)
Substance | C (s) | H2 (g) | CH3COCH3 (l) |
|---|---|---|---|
∆HCө / kJ mol–1 | -394 | -286 | -1821 |
Answer:
Step 1: Write the balanced equation

Step 2:Write the combustion products below the equation

Step 3: Draw downward pointing arrows from each substance to its combustion product

Step 4: Write the appropriate values on the arrows and multiply by the number of moles

Step 5: In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction

ΔHfө = -1182 - 858 + 1821 = -219 kJ mol-1
The sign on -1821 needs reversing as the cycle goes in the opposite direction to the arrow pointing to the combustion products
Calculating ΔHr from ΔHf using Hess’s Law energy cycles
Knowing the enthalpy change of formation, ΔHf, allows us to determine the overall enthalpy change of a reaction, ΔHr
To do this, we follow these steps:
Write the equation for the reaction
Write the elements with the correct number of moles and state symbols underneath
Draw upwards pointing arrows to each compound
Write the appropriate values on the arrows and multiply by the number of moles
In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction
Worked Example
Use the information in the table to calculate the enthalpy change for this reaction:
NH4NO3 (s) + ½C (s) → N2 (g) + 2H2O (g) + ½CO2 (g)
Substance | C (s) | N2 (g) | H2O(g) | CO2 (g) | NH4NO3 (s) |
|---|---|---|---|---|---|
∆Hfө / kJ mol–1 | 0 | 0 | –242 | –394 | –365 |
Answer:
Step 1: Write the balanced equation

Step 2: Write the elements with the correct number of moles and state symbols underneath

Step 3: Draw upwards pointing arrows to each compound

Step 4: Write the appropriate values on the arrows and multiply by the number of moles

Step 5: In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction

ΔHrө = +365 - 484 - 197 = -316 kJ mol-1
The sign on -365 needs reversing as the cycle goes in the opposite direction to the arrow pointing upwards
There is no need to draw arrows from the elements to carbon and nitrogen as ΔHfө is 0 for elements
Calculating average bond energies using Hess’s cycles
Bond energies cannot be found directly so enthalpy cycles are used to find the average bond energy
This can be done using enthalpy changes of atomisation and combustion or formation
The enthalpy change of atomisation (ΔHatꝋ ) is the enthalpy change when one mole of gaseous atoms is formed from its elements under standard conditions.
Eg. ΔHatꝋ [H2] relates to the equation:
½ H2(g) → H(g)
Worked Example
Calculating average C-H bond energy
Calculate the average bond energy of the C-H bond using the relevant ΔHfꝋ and ΔHatꝋ values in the table below:

Answer
Step 1: Write down the equation for the dissociation of methane at the top

Step 2: Write down the elements at the bottom
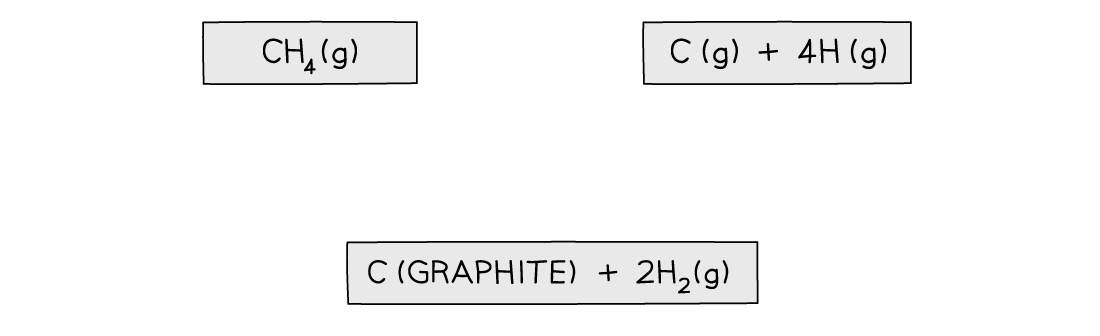
Step 3: Draw all arrows in the correct direction
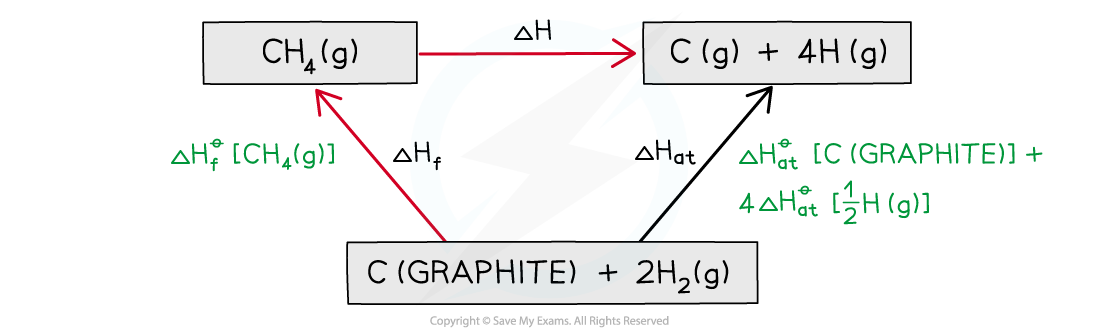
Step 4: Apply Hess’s Law
ΔH = ΔHθat + ΔHθf
ΔH = ((+717.7) + (4 x (+218)) - (-74.8)
ΔH = +1664.5 kJ mol-1
Step 5: Since there are 4 C-H bonds in methane:
Average bond enthalpy (C-H) =
Average bond enthalpy (C-H) = +416.1 kJ mol-1
Examiner Tips and Tricks
Remember: Take into account the number of moles of each reactant and product.
For example, there are two moles of NaHCO3(s) so the ΔHf value is multiplied by 2.

Unlock more, it's free!
Was this revision note helpful?
