Reactions of Alkenes (AQA AS Chemistry): Revision Note
Exam code: 7404
Alkenes: Reactions
Alkenes are very useful compounds as they can undergo many types of reactions
They can therefore be used as starting molecules when making new compounds

Electrophilic addition
Electrophilic addition is the addition of an electrophile to a double bond
The C=C double bond is broken, and a new single bond is formed from each of the two carbon atoms
Electrophilic addition reactions include the addition of:
Steam (H2O (g))
Hydrogen halide (HX)
Halogen

The diagram shows an overview of the different electrophilic addition reactions alkenes can undergo
Electrophilic Addition
Alkenes are more reactive than alkanes due to their carbon-carbon double bonds
The double bond has high electron density, making it susceptible to electrophilic attack
Electrophilic addition occurs when an electrophile adds to the double bond, breaking it and forming new single bonds with each carbon.
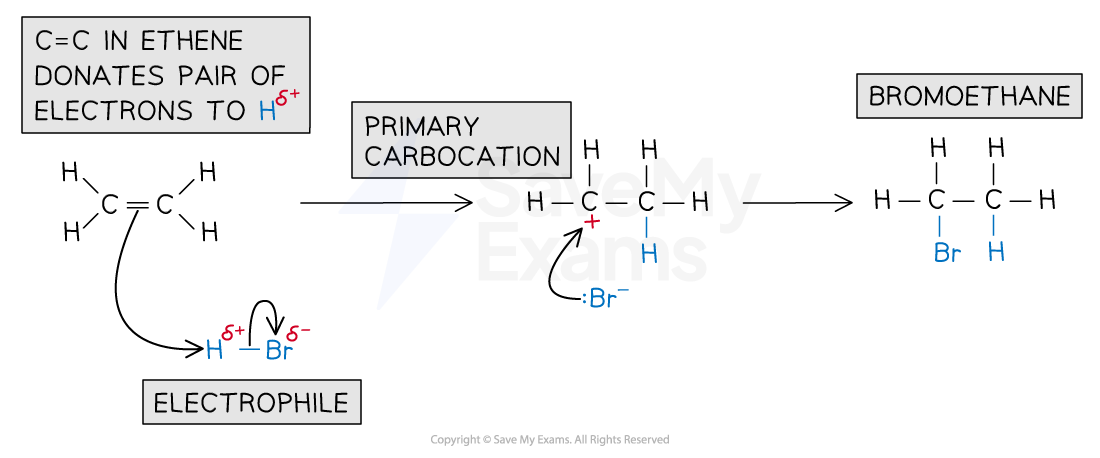
Reaction with HBr
Hydrogen bromide (HBr) is polar due to differences in electronegativity between hydrogen and bromine
Bromine pulls electrons more strongly, creating a partial negative charge on Br and a partial positive charge on H
The polarity of a HBr molecule

The two atoms have different electronegativities resulting in the formation of a polar bond
In electrophilic addition:
The partially positive (δ+) hydrogen atom acts as an electrophile
It is attracted to the high electron density of the C=C double bond in the alkene and accepts a pair of electrons
The H-Br bond breaks heterolytically, forming a Br- ion
A highly reactive carbocation intermediate is formed which reacts with the bromide ion, Br-
The reaction of ethene with HBr forms bromoethane
Electrophilic addition of HBr mechanism
Reaction with Br2
Bromine (Br₂) is non-polar because both atoms share electrons equally
When Br₂ approaches an alkene's double bond, the high electron density repels the electron pair in Br-Br
This causes the Br atom closest to the double bond to become partially positive (δ+), while the other Br atom becomes partially negative (δ-)
The polarity of a Br2 molecule

In an addition reaction:
The closest Br atom acts as an electrophile and accepts a pair of electrons from the C=C bond in the alkene
The Br-Br bond breaks heterolytically, forming a Br- ion
This results in the formation of a highly reactive carbocation intermediate which reacts with the Br- (nucleophile)
The reaction of ethene with Br2 forms 1,2-dibromoethane
Electrophilic addition of Br2 mechanism

Reaction with H2SO4
Alkenes react with concentrated sulphuric acid in the cold to produce alkyl hydrogensulphates
Ethene reacts to give ethyl hydrogensulphate
Concentrated sulfuric acid adds across the double bond
The hydrogen atom in sulfuric acid has a partial positive charge so a sulfuric acid molecule acts as electrophile
The polarity of a H2SO4 molecule

In electrophilic addition:
The partially positive (δ+) hydrogen atom acts as an electrophile
It is attracted to the high electron density of the C=C double bond in the alkene and accepts a pair of electrons
The H-O bond breaks heterolytically, forming a hydrogensulfate ion, HSO4-
A highly reactive carbocation intermediate is formed which reacts with the HSO4-
Electrophilic addition of H2SO4 mechanism

The product formed reacts with water to form an alcohol and sulfuric acid
Essentially, water adds across the double bond with sulfuric acid acting as a catalyst
Examiner Tips and Tricks
The stability of the carbocation intermediate is as follows:
tertiary > secondary > primary
When more than one carbocations can be formed, the major product of the reaction will be the one that results from the nucleophilic attack of the most stable carbocation.

Unlock more, it's free!
Was this revision note helpful?
