The Mechanisms of Electrophilic Addition Reactions (HL) (DP IB Chemistry): Revision Note
The mechanisms of electrophilic addition reactions
Electrophilic addition
Electrophilic addition is a reaction in which an electrophile (or Lewis acid) adds across the C=C double bond of an alkene
The C=C bond is an area of high electron density
This makes it susceptible to attack by electrophiles
The π bond in the double bond breaks, forming:
A single C–C bond
Two new σ-bonds from each carbon atom
Electrophilic addition reactions include the addition of:
Hydrogen, H2 (g) - catalytic hydrogenation
Steam, H2O (g) - hydration of alkenes
Hydrogen halides, HX - formation of halogenoalkanes
Halogens, X2 - formation of dihalogenoalkanes

Examiner Tips and Tricks
The IB Chemistry Guide states that you need to be able to describe and explain the electrophilic addition mechanisms for symmetrical alkenes with:
Halogens
Water
Hydrogen halides
Hydrogenation is assumed prior knowledge, no mechanism is required.
Addition of steam (H2O)
Water is a weak electrophile
Water does not readily react with alkenes without a strong acid catalyst
H3O+ acts as the electrophile
The electrophilic addition of water occurs in two steps:
Step 1
The π electrons in the C=C attack a proton from H3O+
Heterolytic fission occurs and a carbocation is formed
Step 2
Water donates a lone pair of electrons to the carbocation
This forms a protonated alcohol (oxonium ion)
An equilibrium is established between the protonated alcohol and the alcohol
This regenerates the H3O+ catalyst
Electrophilic addition of H2O mechanism

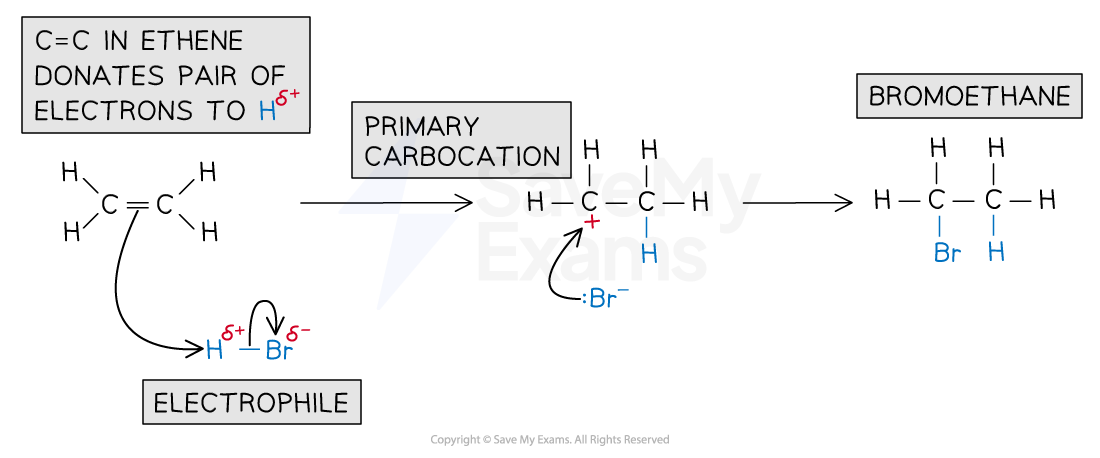
Addition of hydrogen halides (HX)
Hydrogen halides such as HBr are permanently polar
This is due to the electronegativity difference between H and the halogen
In a hydrogen bromide molecule, the bromine atom has a stronger pull on the electrons in the H-Br bond
This causes:
The Br atom to have a partial negative charge
The H atom to have a partial positive charge

The electrophilic addition of hydrogen halides occurs in two steps:
Step 1
The π electrons in the C=C attack the δ⁺ hydrogen atom in HBr
Heterolytic fission occurs forming a carbocation intermediate and a bromide ion
Step 2
The bromide ion donates a lone pair of electrons to the carbocation
This forms the halogenoalkane product
Examiner Tips and Tricks
For electrophilic addition mechanisms, the curly arrows must:
Be double-headed to show the movement of an electron pair
Start from a lone pair or a region of high electron density (e.g. a C=C bond)
Point towards a δ⁺ atom (electrophile) or a positive charge (e.g. carbocation)
Examiners often comment on incorrect or careless use of curly arrows in organic mechanisms
Arrows must be positioned and directed precisely
Addition of halogens (X2)
Halogen molecules like Br2 are non-polar
They become polarised when they approach the high electron density of a C=C bond
This induces a temporary dipole in the Br–Br bond
One Br atom becomes slightly δ⁺ and the other δ⁻

The electrophilic addition of bromine occurs in two steps:
Step 1
The π electrons in the C=C attack the δ⁺ bromine atom in Br2
Heterolytic fission occurs forming a brominated carbocation intermediate and a bromide ion
Step 2
The bromide ion donates a lone pair of electrons to the carbocation
This forms the 1,2-dibromoalkane product

Examiner Tips and Tricks
The electrophilic addition mechanisms for halogens and hydrogen halides are similar
The key difference is that:
Hydrogen halides are permanently polar
Halogens are non-polar and rely on temporary induced dipoles

Unlock more, it's free!
Was this revision note helpful?
