Periodicity - Thermal Trends (Edexcel International AS Chemistry): Revision Note
Exam code: XCH11
Trends in Melting & Boiling Point
Melting point
Period 2 and 3 elements follow the same pattern in relation to their melting points
Melting Points of the Elements Across Period 3 Table

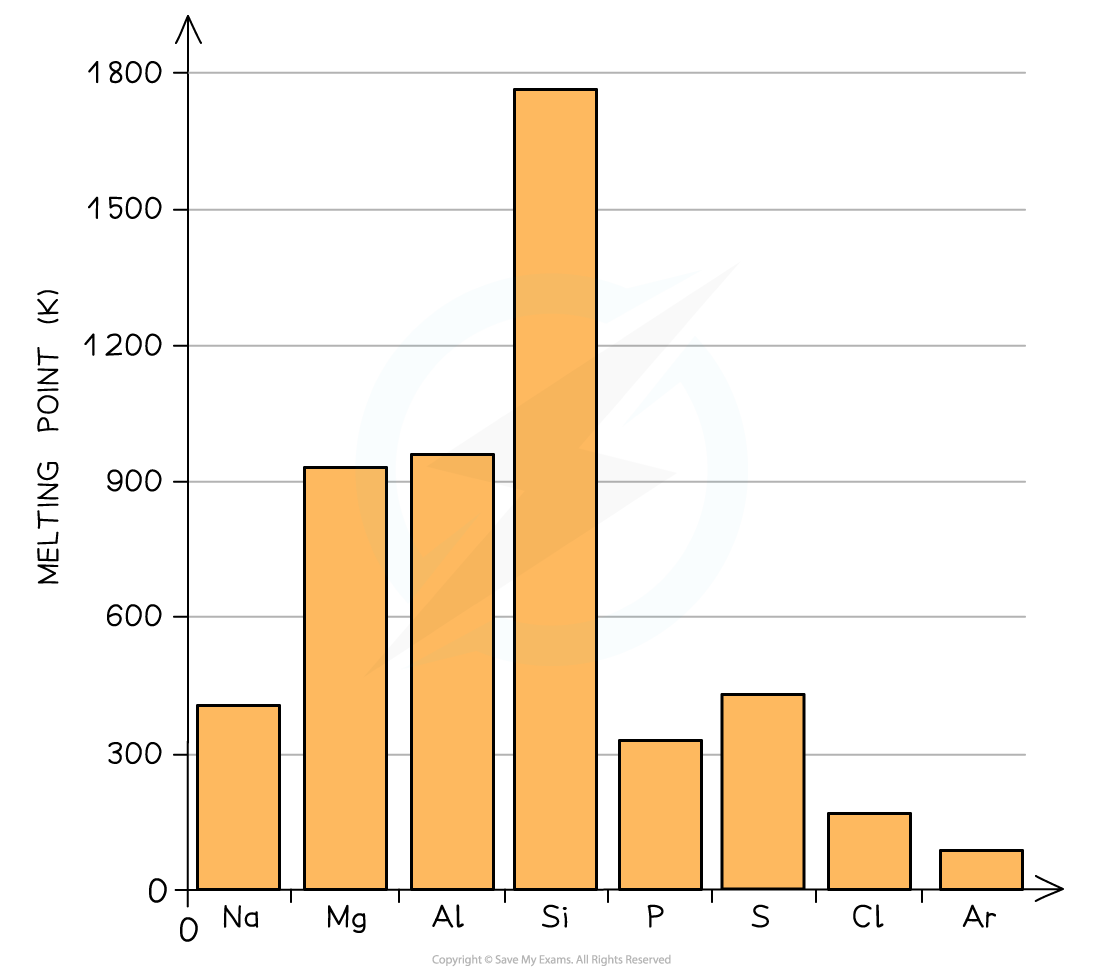
A general increase in melting point for the Period 3 elements up to silicon is observed
Silicon has the highest melting point
After the Si element the melting points of the elements decreases significantly
The above trends can be explained by looking at the bonding and structure of the elements
Bonding & Structure of the Elements Table

The table shows that Na, Mg and Al are metallic elements which form positive ions arranged in a giant lattice in which the ions are held together by a 'sea' of delocalised electrons

Metal cations form a giant lattice held together by electrons that can freely move around
Examiner Tips and Tricks
Remember: At room temperature and pressure, metals (except for mercury) are solid
This means that the lattice structure should:
Have a regular arrangement of positive ions (rows and columns)
Have the ions tightly packed / close together
The lattice structure should not:
Have large gaps between the positive ions
This could lose marks in an exam as examiners may not be satisfied that a solid is being shown
Have a random arrangement of particles
Examiners would consider a randomly arranged, close packed structure to be a liquid and penalise answers accordingly
The delocalised electrons do not have to be specifically shown
The electrons in the ‘sea’ of delocalised electrons are those from the valence shell of the atoms
Na will donate one electron into the ‘sea’ of delocalised electrons, Mg will donate two and Al three electrons
As a result of this, the metallic bonding in Al is stronger than in Na
This is because the electrostatic forces between a 3+ ion and the larger number of negatively charged delocalised electrons is much larger compared to a 1+ ion and the smaller number of delocalised electrons in Na
Because of this, the melting points increase going from Na to Al
Si has the highest melting point due to its giant molecular structure in which each Si atom is held to its neighbouring Si atoms by strong covalent bonds
P, S, Cl and Ar are non-metallic elements and exist as simple molecules (P4, S8, Cl2 and Ar as a single atom)
The covalent bonds within the molecules are strong, however, between the molecules, there are only weak instantaneous dipole-induced dipole forces
It doesn’t take much energy to break these intermolecular forces
Therefore, the melting points decrease going from P to Ar (note that the melting point of S is higher than that of P as sulphur exists as larger S8 molecules compared to the smaller P4 molecule)

Unlock more, it's free!
Was this revision note helpful?
