Nuclear Fusion & Fission (Cambridge (CIE) A Level Physics): Revision Note
Exam code: 9702
Nuclear fusion & fission
Nuclear fusion
Nuclear fusion is when:
Two nuclei combine to form a single nucleus
Low mass nuclei, such as hydrogen and helium, can undergo fusion and release energy
For example, when two hydrogen nuclei (protons) fuse, a deuterium nucleus is produced
A positron and an electron neutrino are also produced as one of the protons converts into a neutron through beta-plus decay
In the centres of stars, four hydrogen nuclei
fuse to produce a helium nucleus
, plus the release of energy
This provides fuel for the star to continue burning
On Earth, research is focused on achieving the deuterium-tritium (D-T) reaction
This involves fusing a deuterium nucleus and a tritium nucleus together to produce a helium nucleus and a neutron
Deuterium-tritium fusion
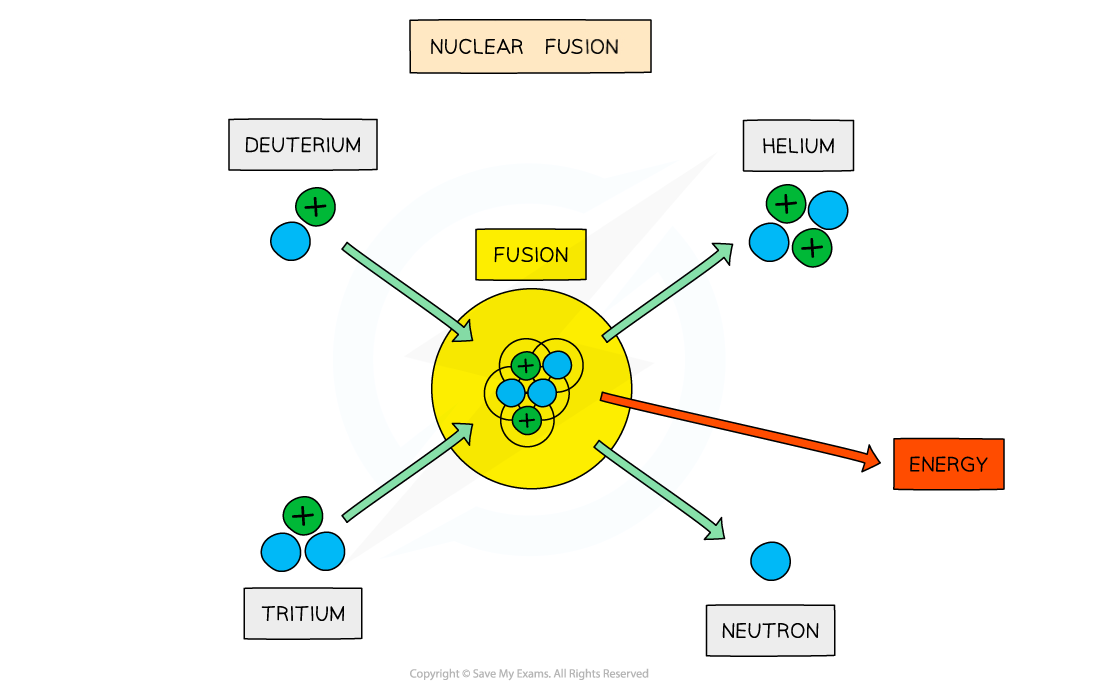
For two nuclei to fuse, both nuclei must have high kinetic energy
This is because nuclei must be able to get close enough to fuse
However, two forces acting within the nuclei make this difficult to achieve
Electrostatic repulsion
Protons inside the nuclei are positively charged, which means that they electrostatically repel one another
Strong nuclear force
The strong nuclear force, which binds nucleons together, acts at very short distances within nuclei
Therefore, nuclei must get very close together for the strong nuclear force to take effect
It takes a great deal of energy to overcome the electrostatic force, hence, fusion can only be achieved in an extremely hot environment, such as the core of a star
Nuclear fission
Nuclear fission is when:
A single large nucleus divides to form smaller nuclei
High mass nuclei (such as uranium) can undergo fission and release energy
Induced fission
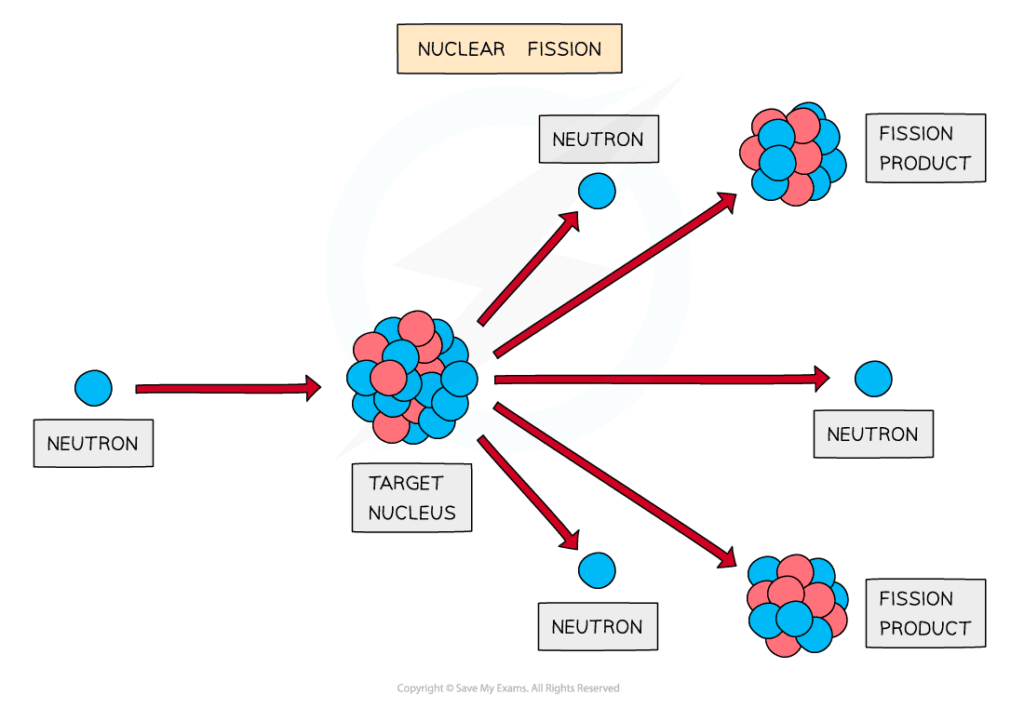
Fission must be induced by firing neutrons at a nucleus
When a neutron strikes a nucleus, it splits into two or more daughter nuclei
During fission, neutrons are ejected from the nucleus, which in turn can collide with other nuclei, triggering a cascade effect
This leads to a chain reaction, which lasts until all of the material has undergone fission, or the reaction is halted by a moderator
Nuclear fission is the process which produces energy in nuclear power stations, where it is well controlled
When nuclear fission is not controlled, the chain reaction can cascade to produce the effects of a nuclear bomb
Examiner Tips and Tricks
When an atom undergoes nuclear fission, take note that extra neutrons are ejected by the nucleus and not from the fission products
Significance of binding energy per nucleon
At low values of A:
attractive nuclear forces between nucleons dominate over repulsive electrostatic forces between protons
in the right conditions, nuclei undergo fusion
In fusion, the mass of the nucleus that is created is slightly less than the total mass of the original nuclei
The mass defect is equal to the binding energy that is released since the nucleus that is formed is more stable
At high values of A:
repulsive electrostatic forces between forces begin to dominate, and these forces tend to break apart the nucleus rather than hold it together
in the right conditions, nuclei undergo fission
In fission, an unstable nucleus is converted into more stable nuclei with a smaller total mass
This difference in mass, the mass defect, is equal to the binding energy that is released

Unlock more, it's free!
Was this revision note helpful?
