The Wave Nature of Electrons (Edexcel A Level Physics): Revision Note
Exam code: 9PH0
The Wave Nature of Electrons
Electron diffraction was the first clear evidence that matter can behave like light and has wave properties
This is demonstrated using the electron diffraction tube
The electrons are accelerated in an electron gun to a high potential, such as 5000 V, and are then directed through a thin film of graphite
The lattice structure of the graphite acts like the slits in a diffraction grating
The electrons diffract from the gaps between carbon atoms and produce a circular pattern on a fluorescent screen made from phosphor
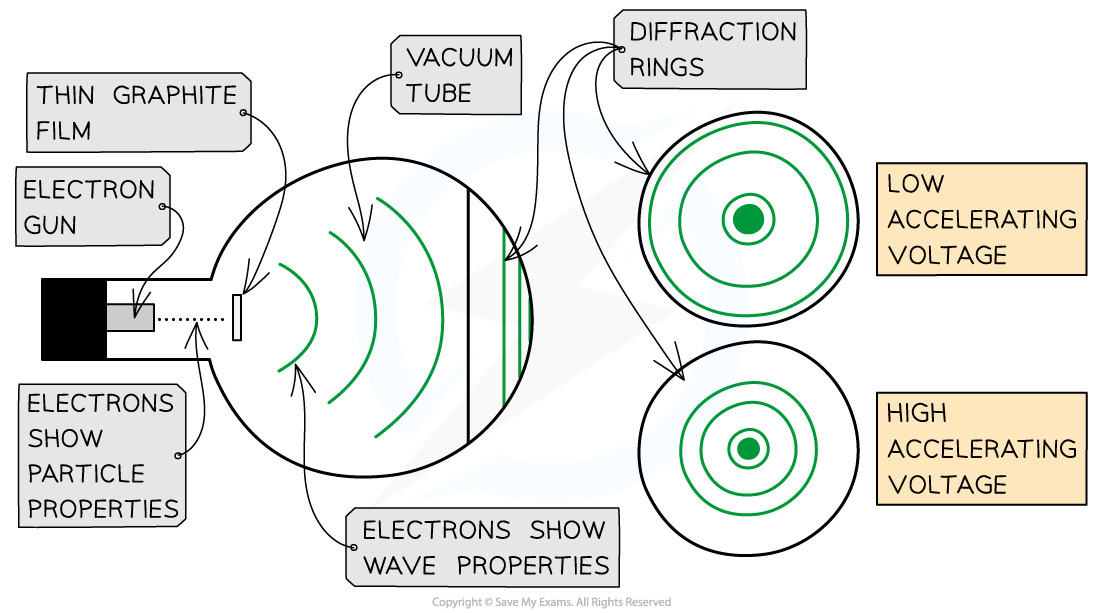
Electrons accelerated through a high potential difference demonstrate wave-particle duality
In order to observe the diffraction of electrons, they must be focused through a gap similar to their size, such as an atomic lattice
Graphite film is ideal for this purpose because of its crystalline structure
The gaps between neighbouring planes of the atoms in the crystals act as slits, allowing the electron waves to spread out and create a diffraction pattern
The diffraction pattern is observed on the screen as a series of concentric rings
This phenomenon is similar to the diffraction pattern produced when light passes through a diffraction grating
If the electrons acted as particles, a pattern would not be observed, instead, the particles would be distributed uniformly across the screen
It is observed that a larger accelerating voltage reduces the diameter of a given ring, while a lower accelerating voltage increases the diameter of the rings

Unlock more, it's free!
Did this page help you?