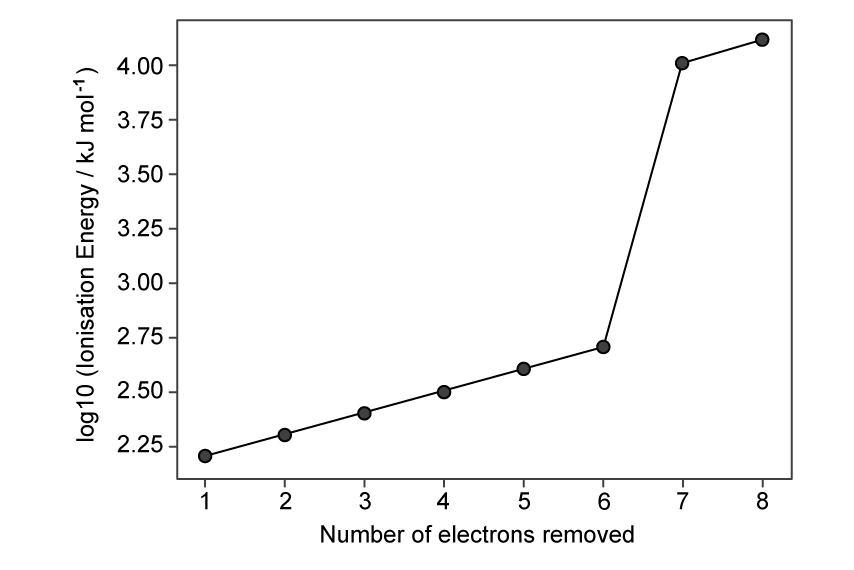
Sodium sulfide, Na2S, is a reactive yellow solid, produced when sodium and sulfur react together.
Use of the periodic table is relevant to this question.
How do the ionic radius and atomic radius of sodium compare with those of sulfur?
| Ionic radius | Atomic radius |
A B C D | Sodium < Sulfur Sodium < Sulfur Sodium > Sulfur Sodium > Sulfur | Sodium < Sulfur Sodium > Sulfur Sodium > Sulfur Sodium < Sulfur |
Was this exam question helpful?