Phospholipids (Cambridge (CIE) AS Biology): Revision Note
Exam code: 9700
The vital role of phospholipids
Structure
Phospholipids are a type of lipid, therefore they are formed from the monomer glycerol and fatty acids
Unlike triglycerides, there are only two fatty acids bonded to a glycerol molecule in a phospholipid as one has been replaced by a phosphate ion (PO43-)
As the phosphate is polar it is soluble in water and described as hydrophilic
The fatty acid ‘tails’ are non-polar and therefore insoluble in water and described as hydrophobic
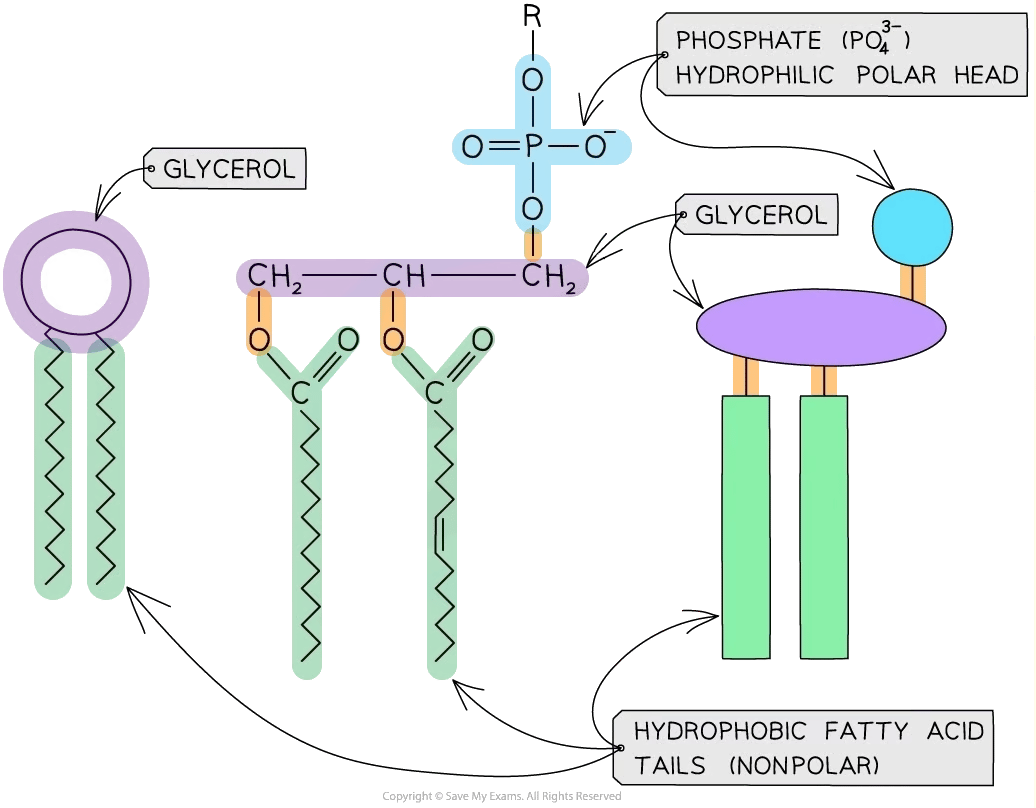
Phospholipids are amphipathic (they have both hydrophobic and hydrophilic parts)
As a result of having hydrophobic and hydrophilic parts phospholipid molecules form monolayers or bilayers in water
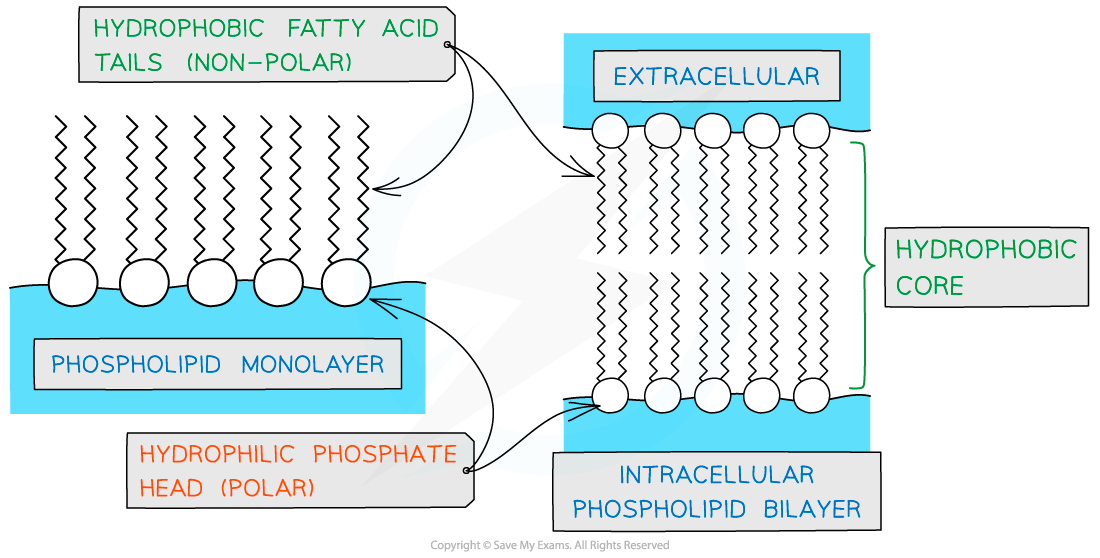
Role of phospholipids
They are the main component (building block) of cell membranes
Due to the presence of hydrophobic fatty acid tails, a hydrophobic core is created when a phospholipid bilayer forms
This acts as a barrier to water-soluble molecules
The hydrophilic phosphate heads form hydrogen bonds with water allowing the cell membrane to be used to compartmentalise
This enables the cells to organise specific roles into organelles helping with efficiency
Composition of phospholipids contributes to the fluidity of the cell membrane
If there are mainly saturated fatty acid tails then the membrane will be less fluid
If there are mainly unsaturated fatty acid tails then the membrane will be more fluid
Phospholipids control membrane protein orientation
Weak hydrophobic interactions between the phospholipids and membrane proteins hold the proteins within the membrane but still allow movement within the layer
Feature | Phospholipid | Triglyceride |
|---|---|---|
Number of fatty acid tails | 2 | 3 |
Presence of phosphate | Yes | No |
Number of ester bonds | 2 | 3 |
Polar or non-polar | Polar phosphate head | Non-polar |
Number of water molecules released during formation | 3 | 3 |
Function | Cell membrane component | Energy storage |
Examiner Tips and Tricks
Ensure you know the difference between phospholipids and triglycerides!

Unlock more, it's free!
Did this page help you?