Nuclear Fusion (Edexcel International A Level (IAL) Physics): Revision Note
Exam code: YPH11
Nuclear Fusion
Fusion is defined as:
Small nuclides that combine together to make larger nuclei, releasing energy
Low mass nuclei, such as hydrogen and helium, can undergo fusion and release energy
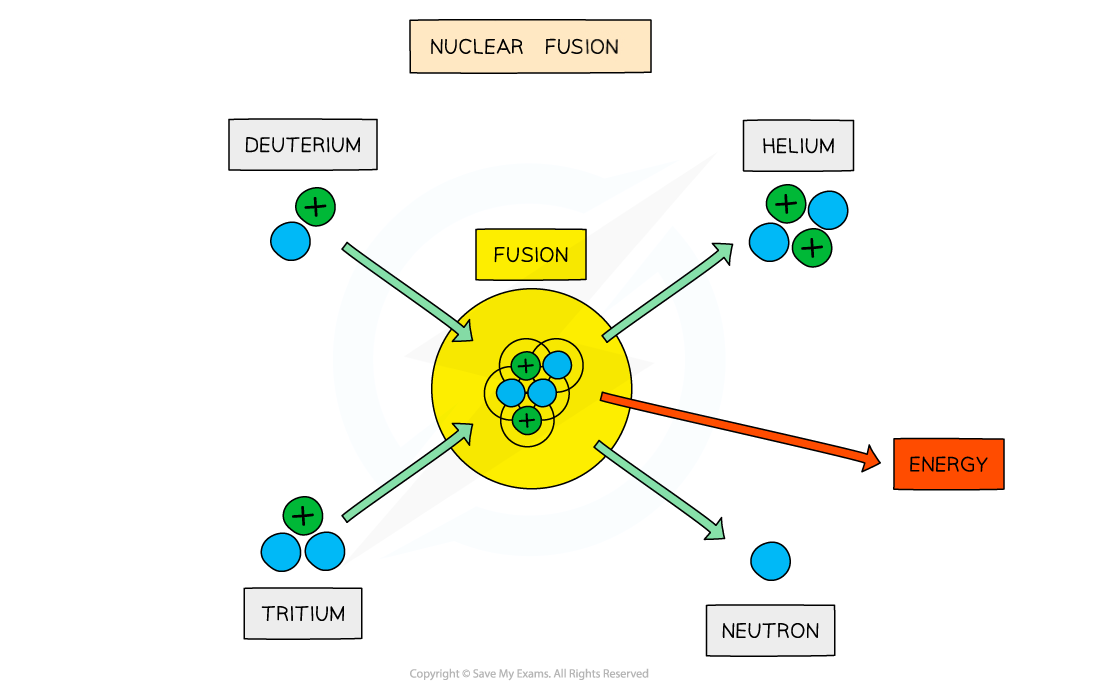
On Earth, research is focused on achieving the deuterium-tritium (D-T) reaction
This involves fusing a deuterium nucleus and a tritium nucleus together to produce a helium nucleus and a neutron
Deuterium-tritium fusion
Conditions for nuclear fusion
For two nuclei to fuse, both nuclei must have high kinetic energy
This is because nuclei must be able to get close enough to fuse
However, two forces acting within the nuclei make this difficult to achieve
Electrostatic repulsion
Protons inside the nuclei are positively charged, which means that they electrostatically repel one another
Strong nuclear force
The strong nuclear force, which binds nucleons together, acts at very short distances within nuclei
Therefore, nuclei must get very close together for the strong nuclear force to take effect
It takes a great deal of energy to overcome the electrostatic force, hence, fusion can only be achieved in an extremely hot, dense environment, such as the core of a star
Fusion products
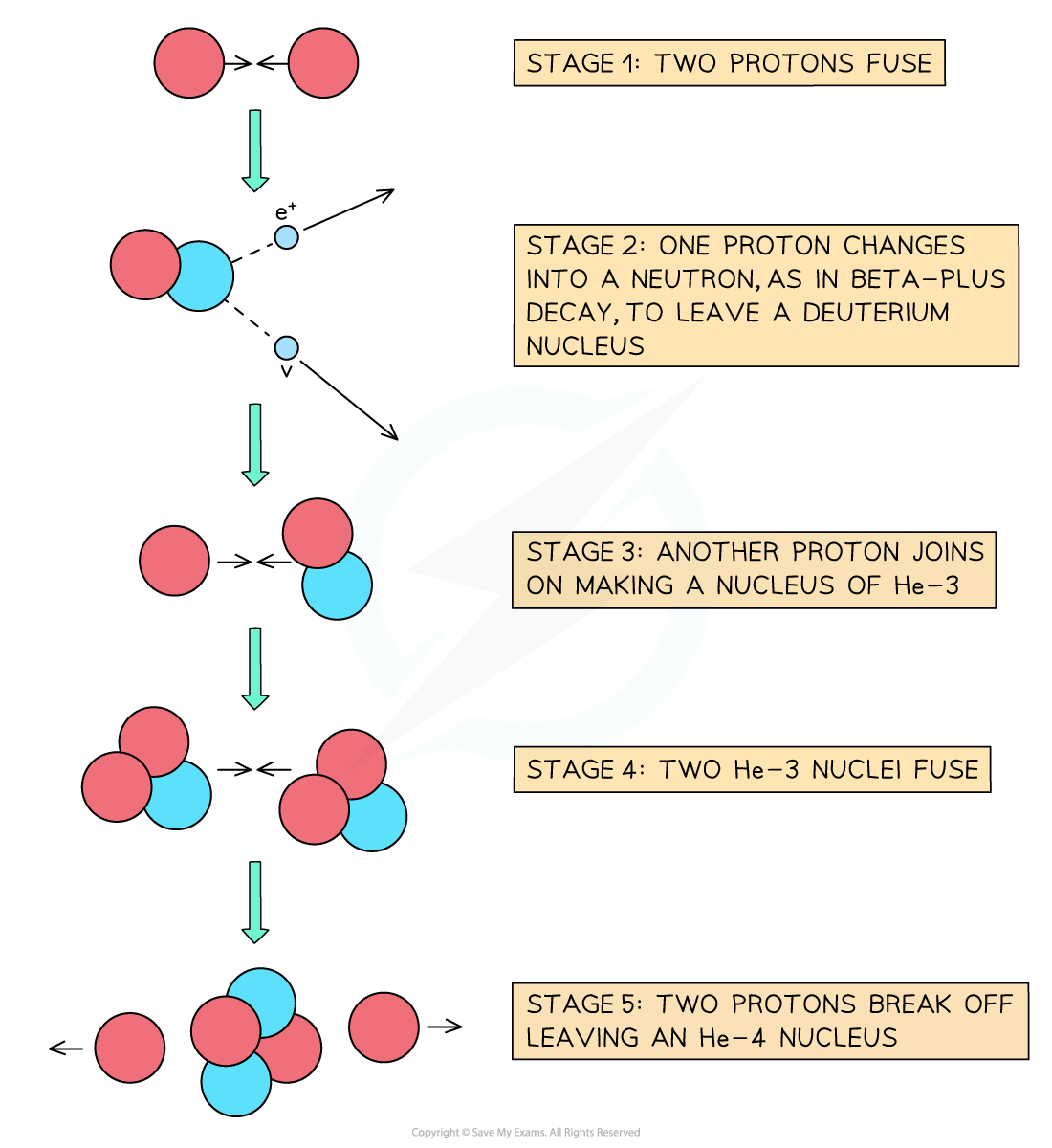
When two hydrogen nuclei (protons) fuse, a deuterium nucleus is produced
A positron and an electron neutrino are also produced as one of the protons converts into a neutron through beta-plus decay
In the centres of stars, four hydrogen nuclei
fuse to produce a helium nucleus
, plus the release of energy
This provides fuel for the star to continue burning
The proton-proton chain
Examiner Tips and Tricks
In the fusion process, the mass of the new, heavier nucleus is less than the mass of the constituent parts of the nuclei fused together, as some mass is converted into energy.
Not all of this energy is used as binding energy for the new, larger nucleus, so energy will be released from this reaction. The binding energy per nucleon afterwards is higher than at the start.

Unlock more, it's free!
Was this revision note helpful?
