Pressure & Temperature in Gases (Cambridge (CIE) O Level Chemistry): Revision Note
Exam code: 5070
Pressure & Temperature in Gases
A change in temperature or pressure affects the volume of gases
As the air inside a hot air balloon is heated up, it expands and the balloon gets bigger
This is because the volume of a gas increases as its temperature increases

As temperature increases gas volume increases. The density decreases as the volume increases so the balloon rises.
If you have a gas stored inside a container that is squeezed, the pressure increases as you decrease the volume
This is what happens in a bicycle pump
As you compress the bicycle pump the high pressure allows you to inflate a tire
You can feel the force of the high pressure if you put your finger on the end of the pump

Pressure increases as volume decreases in a bicycle pump
Kinetic Theory
Gaseous particles are in constant and random motion
The pressure that gas creates inside a closed container is produced by the gaseous particles hitting the inside walls of the container

Moving particles of gas colliding with each other and the container walls
An increase in temperature increases the kinetic energy of each particle, as the heat energy is transformed to kinetic energy, so they move faster
As the temperature increases, the particles in the gas move faster, impacting the container's walls more frequently
If the container walls are flexible and stretchy then the container will get bigger and bigger, just like the hot air balloon!
If the container is made smaller, then the gas particles hit the wall more frequently
So when there is a decrease in volume this causes an increase in gas pressure
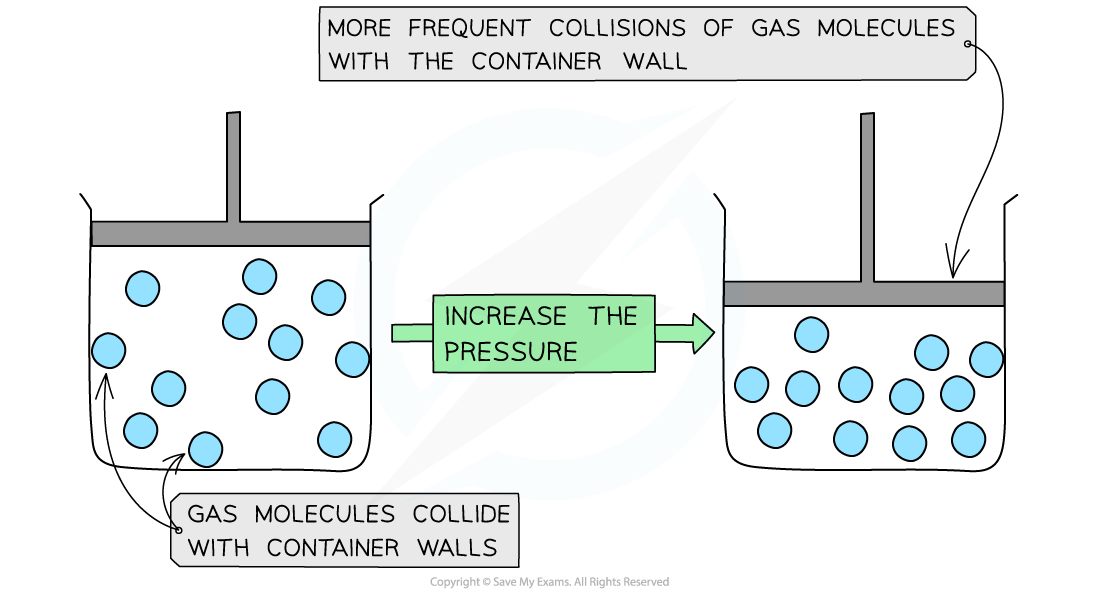
Molecules collide more frequently with the container walls when the pressure is increased

Unlock more, it's free!
Was this revision note helpful?
