Protein Shape (Cambridge (CIE) AS Biology): Revision Note
Exam code: 9700
Proteins: interactions & shape
A polypeptide chain will fold differently due to the interactions (and the bonds that form) between R groups
The three-dimensional configuration that forms is called the tertiary structure of a protein
Each of the twenty amino acids that make up proteins has a unique R group
This means that many different interactions can occur creating a vast range of protein configurations and therefore functions
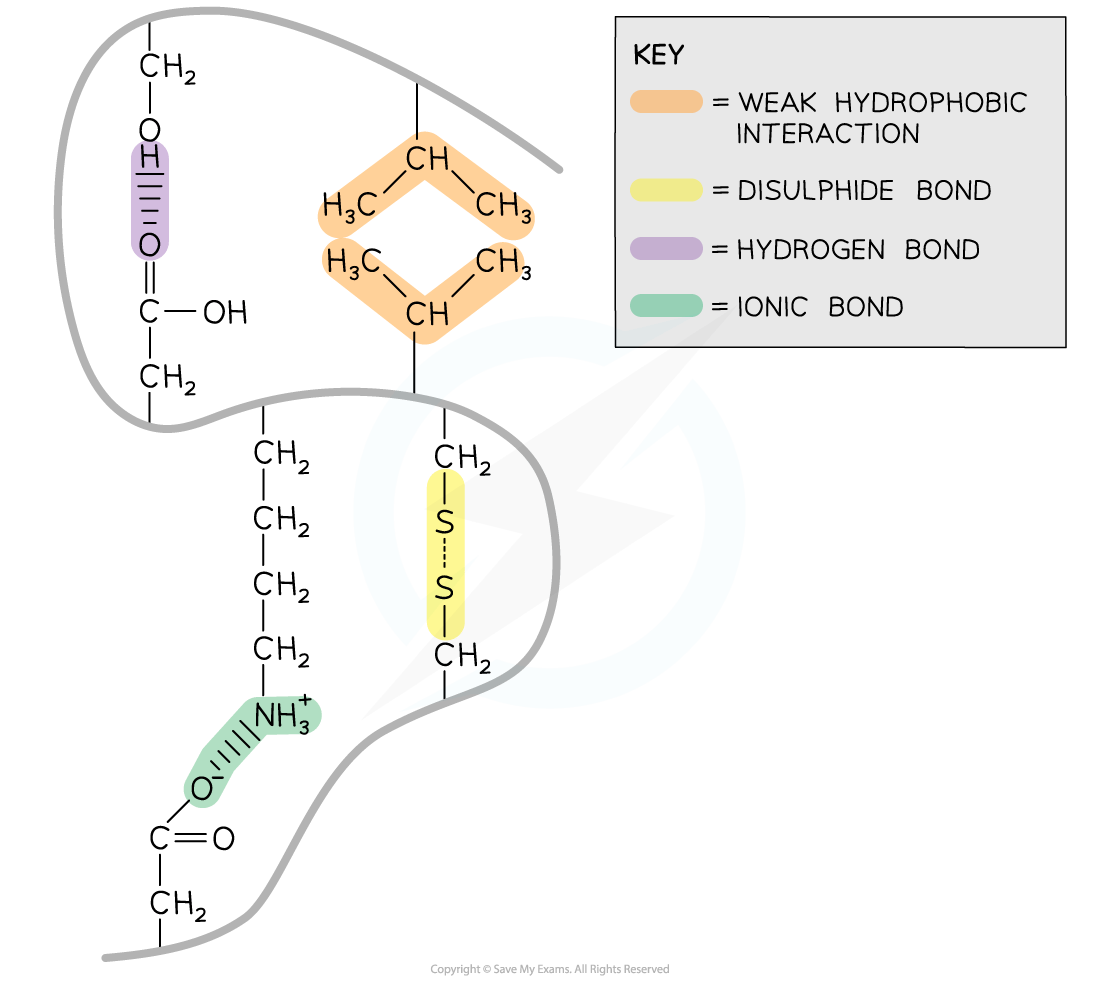
Within proteins with a tertiary structure the following bonds occur:
Strong covalent disulfide
Ionic
Weak hydrogen
Weak hydrophobic interactions
Disulfide
Disulfide bonds are strong covalent bonds that form between two cysteine R groups (as this is the only amino acid with an available sulfur atom in its R group)
These bonds are the strongest within a protein, but occur less frequently, and help stabilise the proteins
These are also known as disulfide bridges
Can be broken by reduction
Disulfide bonds are common in proteins secreted from cells e.g. insulin
Ionic
Ionic bonds form between positively charged (amine group -NH3+) and negatively charged (carboxylic acid -COO-) R groups
Ionic bonds are stronger than hydrogen bonds but they are not common
These bonds are broken by pH changes
Hydrogen
Hydrogen bonds form between strongly polar R groups. These are the weakest bonds that form but the most common as they form between a wide variety of R groups
Hydrophobic interactions
Hydrophobic interactions form between the non-polar (hydrophobic) R groups within the interior of proteins
Examiner Tips and Tricks
Note that an interaction is not the same as a bond. An interaction refers to a low energy attraction between two groups, whereas a bond is high energy and typically involves the electrons of the molecules concerned.
You need to be able to determine which bonds are found in tertiary structures and recognise them in diagrams.

Unlock more, it's free!
Was this revision note helpful?
