Osmosis in Animals (Cambridge (CIE) AS Biology): Revision Note
Exam code: 9700
Osmosis: animal cells
Osmosis is the net movement of water molecules from a region of higher water potential (dilute solution) to a region of lower water potential (concentrated solution), through a selectively permeable membrane
Like plant cells, animal cells can also lose and gain water as a result of osmosis
Because animal cells do not have a supporting cell wall (unlike plant cells), the results of this loss or gain of water on the cell are more severe
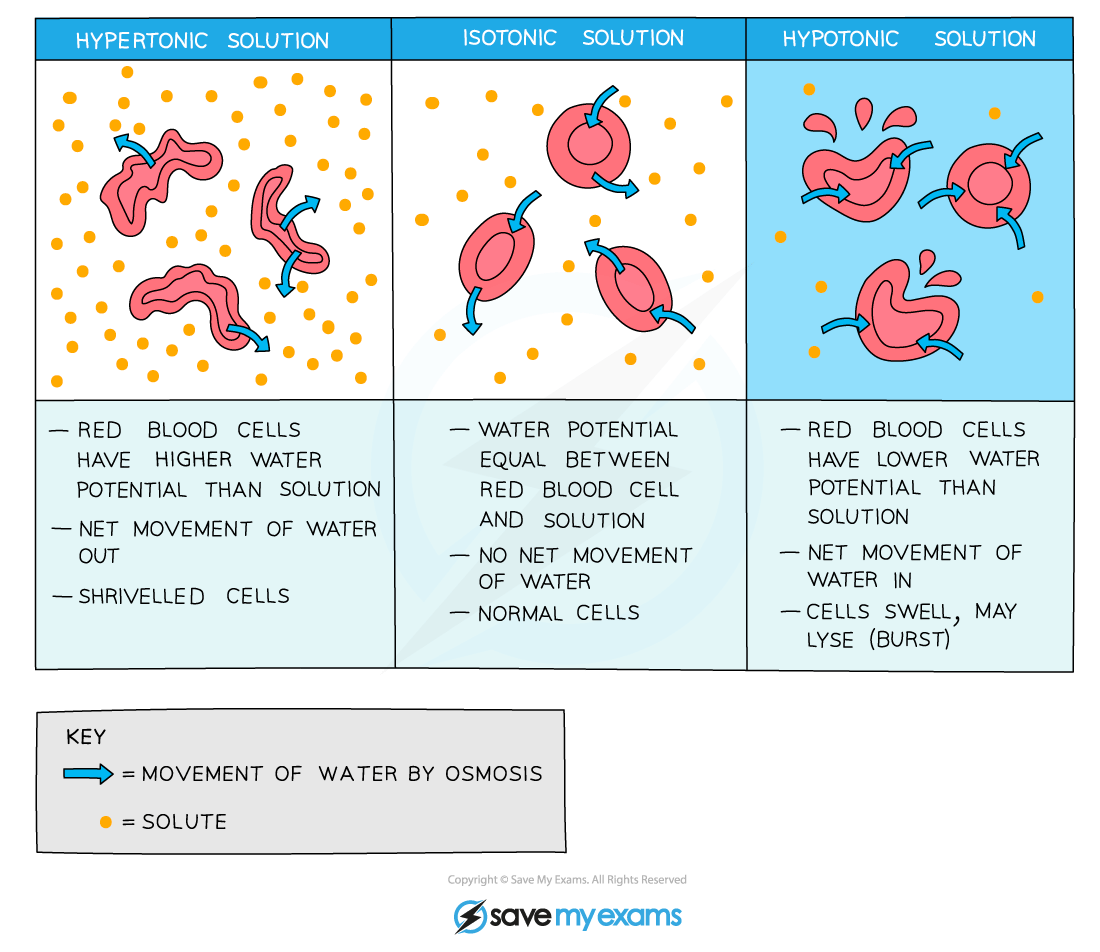
For example, if an animal cell is placed in a solution with a lower water potential than the cell (such as a concentrated sucrose solution), water will leave the cell through its selectively permeable cell surface membrane by osmosis
This will cause the cell to shrink and shrivel up
This occurs when the cell is in a hypertonic environment (the solution outside of the cell has a higher solute concentration than the inside of the cell)
Conversely, if an animal cell is placed in pure water or a dilute solution, water will enter the cell through its selectively permeable cell surface membrane by osmosis, as the pure water or dilute solution has a higher water potential
The cell will continue to gain water by osmosis until the cell membrane is stretched too far and the cell bursts (cytolysis), as it has no cell wall to withstand the increased pressure created
This occurs when the cell is in a hypotonic environment (the solution outside of the cell has a lower solute concentration than the inside of the cell)
This is why a constant water potential must be maintained inside the bodies of animals
If an animal cell is in an isotonic environment (the solution outside of the cell has the same solute concentration as the inside of the cell), the movement of water molecules into and out of the cell occurs at the same rate (no net movement of water)
This means there is no change to the cells
Examiner Tips and Tricks
Be careful with your scientific terminology—animal cells do not plasmolyse because they do not have a cell wall.
In a solution with a lower water potential than the cell itself, animal cells will shrink.
Plasmolysis only occurs in plant cells.

Unlock more, it's free!
Was this revision note helpful?
