Rates of Reaction - Titrimetric Method (Edexcel International A Level (IAL) Chemistry): Revision Note
Exam code: YCH11
Core Practical 9a: Titrimetric Methods
This practical uses a titrimetric method (a technique involving titration) to determine the order of reaction with respect to iodine in its acid-catalysed reaction with propanone
Propanone reacts with iodine in the presence of an acid catalyst:
CH3COCH3 (aq) + I2 (aq) → CH3COCH2I (aq) + H+ (aq) + I- (aq)
Aim
To determine how the concentration of iodine, [I2], affects the rate of the reaction
Experimental design
The concentrations of the other reactants, propanone and H+, do not change
A large excess of both propanone and the acid catalyst is used
This means that their concentrations will remain effectively constant throughout the experiment
Method (sampling and quenching)
We need to find the concentration of iodine at various points in time, so we use a sampling and titration method
Mix 25 cm3 of 1.0 mol dm-3 aqueous propanone with 25 cm3 of 1.0 mol dm-3 sulfuric acid in a beaker
Add 50 cm3 of 0.02 mol dm-3 iodine solution
Start the timer as soon this is added to the beaker
At regular intervals, use a pipette to withdraw 10 cm3 portion of the reaction mixture and transfer this to a conical flask
Quench / stop the sample reacting further by adding a spatula of sodium hydrogen carbonate
Titrate the sample against 0.01 mol dm-3 sodium thiosulfate(VI) solution using starch as an indicator
Starch is used as an indicator is used to determine the concentration of iodine
2S2O32- (aq) + I2 (aq) → 2I- (aq) + S4O62- (aq)
Record the result for each titration in a suitable table
Time (mins) | Titre (cm3) |
|---|---|
0 | |
5 | |
10 |
Practical tips
Have everything ready and set up before beginning the procedure
Keep the timer running throughout the practical rather than stopping the clock
Record the time when the sodium hydrogencarbonate is added to the samples of reaction mixture
This is when the reaction actually stopped
This means that the concentration of iodine can be calculated accurately
Results
Here is an example set of results
Time (mins) | Titre (cm3) |
|---|---|
2.3 | 39.10 |
8.6 | 35.80 |
14.7 | 33.15 |
21.9 | 29.55 |
38.0 | 22.10 |
Analysis and conclusion
Plot a graph of titre (y-axis) against time (x-axis)
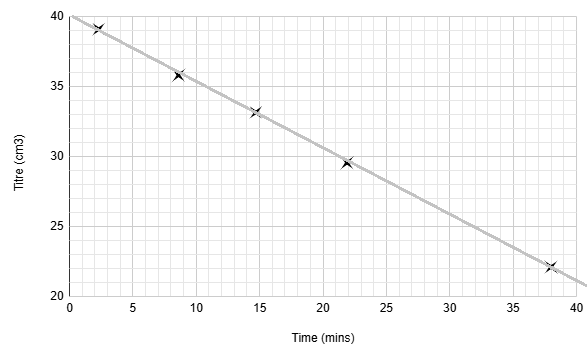
For this reaction, the graph is a straight line with a negative gradient
A straight, descending line on a concentration-time graph means the rate of reaction is constant.
This shows that the rate is independent of the concentration of iodine.
Therefore, the reaction is zero order with respect to iodine

The reaction is first order with respect to propanone and H+
This experiment determines that the reactions is zero order with respect to iodine
So, the overall rate equation is:
Rate = k[CH3COCH3 (aq)] [H+ (aq)]
[I2] does not appear in the rate equation because its order is zero

Unlock more, it's free!
Was this revision note helpful?
