Activity & The Decay Constant (OCR A Level Physics): Revision Note
Exam code: H556
Activity & The Decay Constant
Since radioactive decay is spontaneous and random, it is useful to consider the average number of nuclei which are expected to decay per unit time
This is known as the average decay rate
As a result, each radioactive element can be assigned a decay constant
The decay constant λ is defined as:
The probability, per second, that a given nucleus will decay
When a sample is highly radioactive, this means the number of decays per unit time is very high
This suggests it has a high level of activity
Activity, or the number of decays per unit time can be calculated using:

Where:
A = activity of the sample (Bq)
ΔN = number of decayed nuclei
Δt = time interval (s)
λ = decay constant (s-1)
N = number of nuclei remaining in a sample
The activity of a sample is measured in Becquerels (Bq)
An activity of 1 Bq is equal to one decay per second, or 1 s-1
This equation shows:
The greater the decay constant, the greater the activity of the sample
The activity depends on the number of undecayed nuclei remaining in the sample
The minus sign indicates that the number of nuclei remaining decreases with time - however, for calculations it can be omitted
Worked Example
Americium-241 is an artificially produced radioactive element that emits α-particles. A sample of americium-241 of mass 5.1 μg is found to have an activity of 5.9 × 105 Bq.
(a) Determine the number of nuclei in the sample of americium-241.
(b) Determine the decay constant of americium-241.
Answer:
Part (a)
Step 1: Write down the known quantities
Mass = 5.1 μg = 5.1 × 10-6 g
Molecular mass of americium = 241
NA = Avogadro constant
Step 2: Write down the equation relating number of nuclei, mass and molecular mass
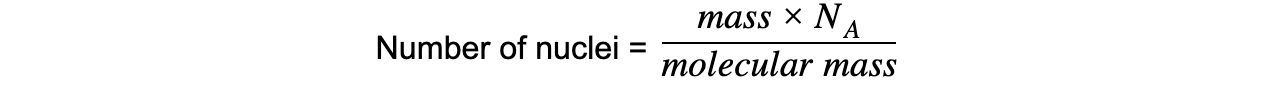
Step 3: Calculate the number of nuclei

Part (b)
Step 1: Write the equation for activity
Activity, A = λN
Step 2: Rearrange for decay constant λ and calculate the answer

Examiner Tips and Tricks
There are lots of new symbols and definitions in this unit. Make sure you are clear on what each symbol means.

Unlock more, it's free!
Was this revision note helpful?
